The regulated expression, intracellular trafficking, and membrane recycling of the P2Y-like receptor GPR17 in Oli-neu oligodendroglial cells
- PMID: 23288840
- PMCID: PMC3576128
- DOI: 10.1074/jbc.M112.404996
The regulated expression, intracellular trafficking, and membrane recycling of the P2Y-like receptor GPR17 in Oli-neu oligodendroglial cells
Abstract
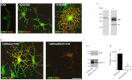
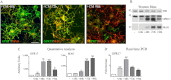
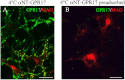
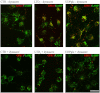
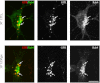
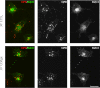
GPR17 is a G-protein-coupled receptor that is activated by two classes of molecules: uracil-nucleotides and cysteinyl-leukotrienes. GPR17 is required for initiating the differentiation of oligodendrocyte precursors but has to be down-regulated to allow cells to undergo terminal maturation. Although a great deal has been learned about GPR17 expression and signaling, no information is currently available about the trafficking of native receptors after the exposure of differentiating oligodendrocytes to endogenous agonists. Here, we demonstrate that neuron-conditioned medium induces the transcriptionally mediated, time-regulated expression of GPR17 in Oli-neu, an oligodendrocyte precursor cell line, making these cells suitable for studying the endocytic traffic of the native receptor. Agonist-induced internalization, intracellular trafficking, and membrane recycling of GPR17 were analyzed by biochemical and immunofluorescence assays using an ad hoc-developed antibody against the extracellular N-terminal of GPR17. Both UDP-glucose and LTD(4) increased GPR17 internalization, although with different efficiency. At early time points, internalized GPR17 co-localized with transferrin receptor, whereas at later times it partially co-localized with the lysosomal marker Lamp1, suggesting that a portion of GPR17 is targeted to lysosomes upon ligand binding. An analysis of receptor recycling and degradation demonstrated that a significant aliquot of GPR17 is recycled to the cell surface. Furthermore, internalized GPR17 displayed a co-localization with the marker of the "short loop" recycling endosomes, Rab4, while showing very minor co-localization with the "long loop" recycling marker, Rab11. Our results provide the first data on the agonist-induced trafficking of native GPR17 in oligodendroglial cells and may have implications for both physiological and pathological myelination.
Figures









Similar articles
-
SNX27, a protein involved in down syndrome, regulates GPR17 trafficking and oligodendrocyte differentiation.Glia. 2016 Aug;64(8):1437-60. doi: 10.1002/glia.23015. Epub 2016 Jun 6. Glia. 2016. PMID: 27270750
-
The Orphan G Protein-coupled Receptor GPR17 Negatively Regulates Oligodendrocyte Differentiation via Gαi/o and Its Downstream Effector Molecules.J Biol Chem. 2016 Jan 8;291(2):705-18. doi: 10.1074/jbc.M115.683953. Epub 2015 Nov 30. J Biol Chem. 2016. PMID: 26620557 Free PMC article.
-
The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair.PLoS One. 2008;3(10):e3579. doi: 10.1371/journal.pone.0003579. Epub 2008 Oct 31. PLoS One. 2008. PMID: 18974869 Free PMC article.
-
The G Protein-Coupled Receptor GPR17: Overview and Update.ChemMedChem. 2016 Dec 6;11(23):2567-2574. doi: 10.1002/cmdc.201600453. Epub 2016 Nov 14. ChemMedChem. 2016. PMID: 27863043 Review.
-
Pharmacological Properties and Biological Functions of the GPR17 Receptor, a Potential Target for Neuro-Regenerative Medicine.Adv Exp Med Biol. 2017;1051:169-192. doi: 10.1007/5584_2017_92. Adv Exp Med Biol. 2017. PMID: 28828731 Review.
Cited by
-
Development of the first in vivo GPR17 ligand through an iterative drug discovery pipeline: A novel disease-modifying strategy for multiple sclerosis.PLoS One. 2020 Apr 22;15(4):e0231483. doi: 10.1371/journal.pone.0231483. eCollection 2020. PLoS One. 2020. PMID: 32320409 Free PMC article.
-
Olig2-Targeted G-Protein-Coupled Receptor Gpr17 Regulates Oligodendrocyte Survival in Response to Lysolecithin-Induced Demyelination.J Neurosci. 2016 Oct 12;36(41):10560-10573. doi: 10.1523/JNEUROSCI.0898-16.2016. J Neurosci. 2016. PMID: 27733608 Free PMC article.
-
Epigenetic priming of immune/inflammatory pathways activation and abnormal activity of cell cycle pathway in a perinatal model of white matter injury.Cell Death Dis. 2022 Dec 13;13(12):1038. doi: 10.1038/s41419-022-05483-4. Cell Death Dis. 2022. PMID: 36513635 Free PMC article.
-
Expression of dual nucleotides/cysteinyl-leukotrienes receptor GPR17 in early trafficking of cardiac stromal cells after myocardial infarction.J Cell Mol Med. 2014 Sep;18(9):1785-96. doi: 10.1111/jcmm.12305. Epub 2014 Jun 7. J Cell Mol Med. 2014. PMID: 24909956 Free PMC article.
-
Analysis of chemokines and receptors expression profile in the myelin mutant taiep rat.Oxid Med Cell Longev. 2015;2015:397310. doi: 10.1155/2015/397310. Epub 2015 Mar 25. Oxid Med Cell Longev. 2015. PMID: 25883747 Free PMC article.
References
-
- Ciana P., Fumagalli M., Trincavelli M. L., Verderio C., Rosa P., Lecca D., Ferrario S., Parravicini C., Capra V., Gelosa P., Guerrini U., Belcredito S., Cimino M., Sironi L., Tremoli E., Rovati G. E., Martini C., Abbracchio M. P. (2006) The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 25, 4615–4627 - PMC - PubMed
-
- Lecca D., Trincavelli M. L., Gelosa P., Sironi L., Ciana P., Fumagalli M., Villa G., Verderio C., Grumelli C., Guerrini U., Tremoli E., Rosa P., Cuboni S., Martini C., Buffo A., Cimino M., Abbracchio M. P. (2008) The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS One 3, e3579. - PMC - PubMed
-
- Pugliese A. M., Trincavelli M. L., Lecca D., Coppi E., Fumagalli M., Ferrario S., Failli P., Daniele S., Martini C., Pedata F., Abbracchio M. P. (2009) Functional characterization of two isoforms of the P2Y-like receptor GPR17. [35S]GTPγS binding and electrophysiological studies in 1321N1 cells. Am. J. Physiol. Cell. Physiol. 297, C1028–C1040 - PubMed
-
- Daniele S., Lecca D., Trincavelli M. L., Ciampi O., Abbracchio M. P., Martini C. (2010) Regulation of PC12 cell survival and differentiation by the new P2Y-like receptor GPR17. Cell. Signal. 22, 697–706 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

