Mouse marginal zone B cells harbor specificities similar to human broadly neutralizing HIV antibodies
- PMID: 23288906
- PMCID: PMC3557101
- DOI: 10.1073/pnas.1213713110
Mouse marginal zone B cells harbor specificities similar to human broadly neutralizing HIV antibodies
Abstract
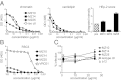
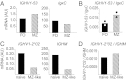
A series of potent, broadly neutralizing HIV antibodies have been isolated from B cells of HIV-infected individuals. VRC01 represents a subset of these antibodies that mediate neutralization with a restricted set of IGHV genes. The memory B cells expressing these antibodies were isolated years after infection; thus, the B-cell subpopulation from which they originated and the extent of participation in the initial HIV antibody response, if any, are unclear. Here we evaluated the frequency of anti-gp120 B cells in follicular (FO) and marginal zone (MZ) B-cell compartments of naïve WT mice and comparable human populations in uninfected individuals. We found that in non-HIV-exposed humans and mice, the majority of gp120-reactive B cells are of naïve and FO phenotype, respectively. Murine FO B cells express a diverse antibody repertoire to recognize gp120. In contrast, mouse MZ B cells recognize gp120 less frequently but preferentially use IGHV1-53 to encode gp120-specific antibodies. Notably, IGHV1-53 shows high identity to human IGHV1-2*02, which has been repeatedly found to encode broadly neutralizing mutated HIV antibodies, such as VRC01. Finally, we show that human MZ-like B cells express IGHV1-2*02, and that IGHV1-53 expression is enriched in mouse MZ B cells. These data suggest that efforts toward developing an HIV vaccine might consider eliciting protective HIV antibody responses selectively from alternative B-cell populations harboring IGHV gene segments capable of producing protective antibodies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Johnston MI, Fauci AS. An HIV vaccine—evolving concepts. N Engl J Med. 2007;356(20):2073–2081. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI052310/AI/NIAID NIH HHS/United States
- AI052157/AI/NIAID NIH HHS/United States
- T32-AI07405/AI/NIAID NIH HHS/United States
- R01 AI097265/AI/NIAID NIH HHS/United States
- HD059527/HD/NICHD NIH HHS/United States
- AI078468/AI/NIAID NIH HHS/United States
- R01 HD059527/HD/NICHD NIH HHS/United States
- T32 AI007405/AI/NIAID NIH HHS/United States
- R56 AI052310/AI/NIAID NIH HHS/United States
- R21 AI078468/AI/NIAID NIH HHS/United States
- R01 AI052310/AI/NIAID NIH HHS/United States
- R01 AI052157/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

