Developmental analysis and influence of genetic background on the Lhx3 W227ter mouse model of combined pituitary hormone deficiency disease
- PMID: 23288907
- PMCID: PMC3548188
- DOI: 10.1210/en.2012-1790
Developmental analysis and influence of genetic background on the Lhx3 W227ter mouse model of combined pituitary hormone deficiency disease
Abstract
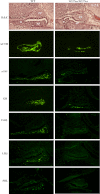
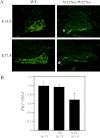
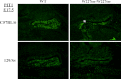
Combined pituitary hormone deficiency (CPHD) diseases result in severe outcomes for patients including short stature, developmental delays, and reproductive deficiencies. Little is known about their etiology, especially the developmental profiles and the influences of genetic background on disease progression. Animal models for CPHD provide valuable tools to investigate disease mechanisms and inform diagnostic and treatment protocols. Here we examined hormone production during pituitary development and the influence of genetic background on phenotypic severity in the Lhx3(W227ter/W227ter) mouse model. Lhx3(W227ter/W227ter) embryos have deficiencies of ACTH, α-glycoprotein subunit, GH, PRL, TSHβ, and LHβ during prenatal development. Furthermore, mutant mice have significant reduction in the critical pituitary transcriptional activator-1 (PIT1). Through breeding, the Lhx3(W227ter/W227ter) genotype was placed onto the 129/Sv and C57BL/6 backgrounds. Intriguingly, the genetic background significantly affected viability: whereas Lhx3(W227ter/W227ter) animals were found in the expected frequencies in C57BL/6, homozygous animals were not viable in the 129/Sv genetic environment. The hormone marker and PIT1 reductions observed in Lhx3(W227ter/W227ter) mice on a mixed background were also seen in the separate strains but in some cases were more severe in 129/Sv. To further characterize the molecular changes in diseased mice, we conducted a quantitative proteomic analysis of pituitary proteins. This showed significantly lower levels of PRL, pro-opiomelanocortin (ACTH), and α-glycoprotein subunit proteins in Lhx3(W227ter/W227ter) mice. Together, these data show that hormone deficiency disease is apparent in early prenatal stages in this CPHD model system. Furthermore, as is noted in human disease, genetic background significantly impacts the phenotypic outcome of these monogenic endocrine diseases.
Figures



References
-
- Mollard P, Hodson DJ, Lafont C, Rizzoti K, Drouin J. A tridimensional view of pituitary development and function. Trends Endocrinol Metab. 2012;23:261–269 - PubMed
-
- Prince KL, Walvoord EC, Rhodes SJ. The role of homeodomain transcription factors in heritable pituitary disease. Nat Rev Endocrinol. 2011;7:727–737 - PubMed
-
- Sharma K, Sheng HZ, Lettieri K, et al. LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons. Cell. 1998;95:817–828 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

