Local experience-dependent changes in the wake EEG after prolonged wakefulness
- PMID: 23288972
- PMCID: PMC3524543
- DOI: 10.5665/sleep.2302
Local experience-dependent changes in the wake EEG after prolonged wakefulness
Abstract
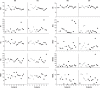
Study objectives: Prolonged wakefulness leads to a progressive increase in sleep pressure, reflected in a global increase in slow wave activity (SWA, 0.5-4.5 Hz) in the sleep electroencephalogram (EEG). A global increase in wake theta activity (5-9 Hz) also occurs. Recently, it was shown that prolonged wakefulness in rodents leads to signs of "local sleep" in an otherwise awake brain, accompanied by a slow/theta wave (2-6 Hz) in the local EEG that occurs at different times in different cortical areas. Compelling evidence in animals and humans also indicates that sleep is locally regulated by the amount of experience-dependent plasticity. Here, we asked whether the extended practice of tasks that involve specific brain circuits results in increased occurrence of local intermittent theta waves in the human EEG, above and beyond the global EEG changes previously described.
Design: Participants recorded with high-density EEG completed 2 experiments during which they stayed awake ≥ 24 h practicing a language task (audiobook listening [AB]) or a visuomotor task (driving simulator [DS]).
Setting: Sleep laboratory.
Patients or participants: 16 healthy participants (7 females).
Interventions: Two extended wake periods.
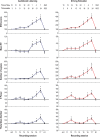
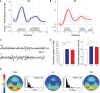
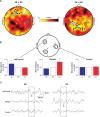
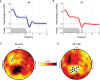
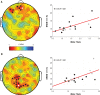
Measurements and results: Both conditions resulted in global increases in resting wake EEG theta power at the end of 24 h of wake, accompanied by increased sleepiness. Moreover, wake theta power as well as the occurrence and amplitude of theta waves showed regional, task-dependent changes, increasing more over left frontal derivations in AB, and over posterior parietal regions in DS. These local changes in wake theta power correlated with similar local changes in sleep low frequencies including SWA.
Conclusions: Extended experience-dependent plasticity of specific circuits results in a local increase of the wake theta EEG power in those regions, followed by more intense sleep, as reflected by SWA, over the same areas.
Figures
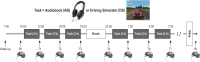
References
-
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–8. - PubMed
-
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

