Nitrogenase: a draft mechanism
- PMID: 23289741
- PMCID: PMC3578145
- DOI: 10.1021/ar300267m
Nitrogenase: a draft mechanism
Abstract
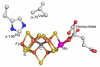
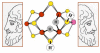
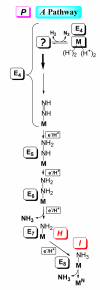
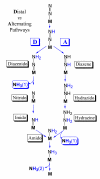
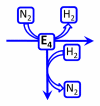
Biological nitrogen fixation, the reduction of N(2) to two NH(3) molecules, supports more than half the human population. The predominant form of the enzyme nitrogenase, which catalyzes this reaction, comprises an electron-delivery Fe protein and a catalytic MoFe protein. Although nitrogenase has been studied extensively, the catalytic mechanism has remained unknown. At a minimum, a mechanism must identify and characterize each intermediate formed during catalysis and embed these intermediates within a kinetic framework that explains their dynamic interconversion. The Lowe-Thorneley (LT) model describes nitrogenase kinetics and provides rate constants for transformations among intermediates (denoted E(n), where n is the number of electrons (and protons), that have accumulated within the MoFe protein). Until recently, however, research on purified nitrogenase had not characterized any E(n) state beyond E(0). In this Account, we summarize the recent characterization of three freeze-trapped intermediate states formed during nitrogenase catalysis and place them within the LT kinetic scheme. First we discuss the key E(4) state, which is primed for N(2) binding and reduction and which we refer to as the "Janus intermediate" because it lies halfway through the reaction cycle. This state has accumulated four reducing equivalents stored as two [Fe-H-Fe] bridging hydrides bound to the active-site iron-molybdenum cofactor ([7Fe-9S-Mo-C-homocitrate]; FeMo-co) at its resting oxidation level. The other two trapped intermediates contain reduced forms of N(2). One, intermediate, designated I, has S = 1/2 FeMo-co. Electron nuclear double resonance/hyperfine sublevel correlation (ENDOR/HYSCORE) measurements indicate that I is the final catalytic state, E(8), with NH(3) product bound to FeMo-co at its resting redox level. The other characterized intermediate, designated H, has integer-spin FeMo-co (non-Kramers; S ≥ 2). Electron spin echo envelope modulation (ESEEM) measurements indicate that H contains the [-NH(2)] fragment bound to FeMo-co and therefore corresponds to E(7). These assignments in the context of previous studies imply a pathway in which (i) N(2) binds at E(4) with liberation of H(2), (ii) N(2) is promptly reduced to N(2)H(2), (iii) the two N's are reduced in two steps to form hydrazine-bound FeMo-co, and (iv) two NH(3) are liberated in two further steps of reduction. This proposal identifies nitrogenase as following a "prompt-alternating (P-A)" reaction pathway and unifies the catalytic pathway with the LT kinetic framework. However, the proposal does not incorporate one of the most puzzling aspects of nitrogenase catalysis: obligatory generation of H(2) upon N(2) binding that apparently "wastes" two reducing equivalents and thus 25% of the total energy supplied by the hydrolysis of ATP. Because E(4) stores its four accumulated reducing equivalents as two bridging hydrides, we propose an answer to this puzzle based on the organometallic chemistry of hydrides and dihydrogen. We propose that H(2) release upon N(2) binding involves reductive elimination of two hydrides to yield N(2) bound to doubly reduced FeMo-co. Delivery of the two available electrons and two activating protons yields cofactor-bound diazene, in agreement with the P-A scheme. This keystone completes a draft mechanism for nitrogenase that both organizes the vast body of data on which it is founded and serves as a basis for future experiments.
Figures

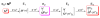



References
-
- Smil V. Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production. MIT Press; Cambridge, MA: 2001.
-
- Eady RR. Structure-function relationships of alternative nitrogenases. Chem. Rev. 1996;96:3013–3030. - PubMed
-
- Burgess BK, Lowe DJ. Mechanism of molybdenum nitrogenase. Chem. Rev. 1996;96:2983–3011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

