Decreased expression of ADAMTS-1 in human breast tumors stimulates migration and invasion
- PMID: 23289900
- PMCID: PMC3600045
- DOI: 10.1186/1476-4598-12-2
Decreased expression of ADAMTS-1 in human breast tumors stimulates migration and invasion
Abstract
Background: ADAMTS-1 (a disintegrin and metalloprotease with thrombospondin motifs) is a member of the ADAMTS family of metalloproteases. Here, we investigated mRNA and protein levels of ADAMTS-1 in normal and neoplastic tissues using qPCR, immunohistochemistry and immunoblot analyses, and we addressed the role of ADAMTS-1 in regulating migration, invasion and invadopodia formation in breast tumor cell lines.
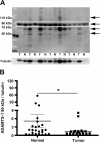
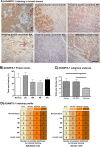
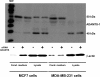
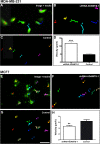
Results: In a series of primary breast tumors, we observed variable levels of ADAMTS-1 mRNA expression but lower levels of ADAMTS-1 protein expression in human breast cancers as compared to normal tissue, with a striking decrease observed in high-malignancy cases (triple-negative for estrogen, progesterone and Her-2). This result prompted us to analyze the effect of ADAMTS-1 knockdown in breast cancer cells in vitro. MDA-MB-231 cells with depleted ADAMTS-1 expression demonstrated increased migration, invasion and invadopodia formation. The regulatory mechanisms underlying the effects of ADAMTS-1 may be related to VEGF, a growth factor involved in migration and invasion. MDA-MB-231 cells with depleted ADAMTS-1 showed increased VEGF concentrations in conditioned medium capable of inducing human endothelial cells (HUVEC) tubulogenesis. Furthermore, expression of the VEGF receptor (VEGFR2) was increased in MDA-MB-231 cells as compared to MCF7 cells. To further determine the relationship between ADAMTS-1 and VEGF regulating breast cancer cells, MDA-MB-231 cells with reduced expression of ADAMTS-1 were pretreated with a function-blocking antibody against VEGF and then tested in migration and invasion assays; both were partially rescued to control levels.
Conclusions: ADAMTS-1 expression was decreased in human breast tumors, and ADAMTS-1 knockdown stimulated migration, invasion and invadopodia formation in breast cancer cells in vitro. Therefore, this series of experiments suggests that VEGF is involved in the effects mediated by ADAMTS-1 in breast cancer cells.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

