Metal-catalyzed oxidation of protein methionine residues in human parathyroid hormone (1-34): formation of homocysteine and a novel methionine-dependent hydrolysis reaction
- PMID: 23289936
- PMCID: PMC3691993
- DOI: 10.1021/mp300563m
Metal-catalyzed oxidation of protein methionine residues in human parathyroid hormone (1-34): formation of homocysteine and a novel methionine-dependent hydrolysis reaction
Abstract
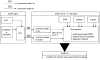
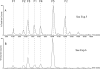
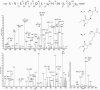
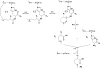
The oxidation of PTH(1-34) catalyzed by ferrous ethylenediaminetetraacetic acid (EDTA) is site-specific. The oxidation of PTH(1-34) is localized primarily to the residues Met[8] and His[9]. Beyond the transformation of Met[8] and His[9] into methionine sulfoxide and 2-oxo-histidine, respectively, we observed a hydrolytic cleavage between Met[8] and His[9]. This hydrolysis requires the presence of Fe(II) and oxygen and can be prevented by diethylenetriaminepentaacetic acid (DTPA) and phosphate buffer. Conditions leading to this site-specific hydrolysis also promote the transformation of Met[8] into homocysteine, indicating that the hydrolysis and transformation of homocysteine may proceed through a common intermediate.
Figures












Similar articles
-
Methionine, tryptophan, and histidine oxidation in a model protein, PTH: mechanisms and stabilization.J Pharm Sci. 2009 Dec;98(12):4485-500. doi: 10.1002/jps.21746. J Pharm Sci. 2009. PMID: 19455640
-
Site-Specific Hydrolysis Reaction C-Terminal of Methionine in Met-His during Metal-Catalyzed Oxidation of IgG-1.Mol Pharm. 2016 Apr 4;13(4):1317-28. doi: 10.1021/acs.molpharmaceut.5b00944. Epub 2016 Mar 11. Mol Pharm. 2016. PMID: 26942274
-
Methionine ligation strategy in the biomimetic synthesis of parathyroid hormones.Biopolymers. 1998 Oct 15;46(5):319-27. doi: 10.1002/(SICI)1097-0282(19981015)46:5<319::AID-BIP3>3.0.CO;2-S. Biopolymers. 1998. PMID: 9754028
-
Oxidation of methionine residues of proteins: biological consequences.Antioxid Redox Signal. 2003 Oct;5(5):577-82. doi: 10.1089/152308603770310239. Antioxid Redox Signal. 2003. PMID: 14580313 Review.
-
Oxidation of methionyl residues in proteins: tools, targets, and reversal.Free Radic Biol Med. 1995 Jan;18(1):93-105. doi: 10.1016/0891-5849(94)00158-g. Free Radic Biol Med. 1995. PMID: 7896176 Review.
Cited by
-
Effect of Iron Oxide Nanoparticles on the Oxidation and Secondary Structure of Growth Hormone.J Pharm Sci. 2019 Oct;108(10):3372-3381. doi: 10.1016/j.xphs.2019.06.007. Epub 2019 Jun 16. J Pharm Sci. 2019. PMID: 31216451 Free PMC article.
-
An Efficient and Rapid Method to Monitor the Oxidative Degradation of Protein Pharmaceuticals: Probing Tyrosine Oxidation with Fluorogenic Derivatization.Pharm Res. 2017 Jul;34(7):1428-1443. doi: 10.1007/s11095-017-2159-6. Epub 2017 Apr 18. Pharm Res. 2017. PMID: 28421307
-
Identifying a role for the interaction of homocysteine and copper in promoting cardiovascular-related damage.Amino Acids. 2021 May;53(5):739-744. doi: 10.1007/s00726-021-02979-9. Epub 2021 Apr 22. Amino Acids. 2021. PMID: 33886000
-
Demethylation of methionine and keratin damage in human hair.Amino Acids. 2018 May;50(5):537-546. doi: 10.1007/s00726-018-2545-3. Epub 2018 Feb 26. Amino Acids. 2018. PMID: 29480334 Free PMC article.
-
A Comprehensive Assessment of All-Oleate Polysorbate 80: Free Fatty Acid Particle Formation, Interfacial Protection and Oxidative Degradation.Pharm Res. 2021 Mar;38(3):531-548. doi: 10.1007/s11095-021-03021-z. Epub 2021 Mar 12. Pharm Res. 2021. PMID: 33713012
References
-
- Cederbaum S, Vilain E. Defects in energy metabolism: coming of age, slowly. J. Pediatr. 2000;136(2):147–8. - PubMed
-
- Stadtman ER. Protein modification in aging. J. Gerontol. 1988;43(5):B112–20. - PubMed
-
- Ozben T, Chevion M. Frontiers in Neurodegenerative Disorders and Aging: Fundamental Aspects, Clinical Perspectives and New Insights. NATO Science Series ed. IOS Press; Amsterdam: 2004.
-
- Manning MC, Chou DK, Murphy BM, Payne RW, Katayama DS. Stability of protein pharmaceuticals: an update. Pharm. Res. 2010;27(4):544–575. - PubMed
-
- Toennies G, Callan TP. Methionine studies: III. A comparison of oxidative reactions of methionine, cysteine, and cystine. Determination of methionine by hydrogen peroxide oxidation. J. Biol. Chem. 1939;129(2):481–490.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

