Metal-catalyzed oxidation of protein methionine residues in human parathyroid hormone (1-34): formation of homocysteine and a novel methionine-dependent hydrolysis reaction
- PMID: 23289936
- PMCID: PMC3691993
- DOI: 10.1021/mp300563m
Metal-catalyzed oxidation of protein methionine residues in human parathyroid hormone (1-34): formation of homocysteine and a novel methionine-dependent hydrolysis reaction
Abstract
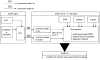
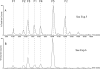
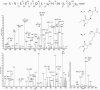
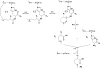
The oxidation of PTH(1-34) catalyzed by ferrous ethylenediaminetetraacetic acid (EDTA) is site-specific. The oxidation of PTH(1-34) is localized primarily to the residues Met[8] and His[9]. Beyond the transformation of Met[8] and His[9] into methionine sulfoxide and 2-oxo-histidine, respectively, we observed a hydrolytic cleavage between Met[8] and His[9]. This hydrolysis requires the presence of Fe(II) and oxygen and can be prevented by diethylenetriaminepentaacetic acid (DTPA) and phosphate buffer. Conditions leading to this site-specific hydrolysis also promote the transformation of Met[8] into homocysteine, indicating that the hydrolysis and transformation of homocysteine may proceed through a common intermediate.
Figures












References
-
- Cederbaum S, Vilain E. Defects in energy metabolism: coming of age, slowly. J. Pediatr. 2000;136(2):147–8. - PubMed
-
- Stadtman ER. Protein modification in aging. J. Gerontol. 1988;43(5):B112–20. - PubMed
-
- Ozben T, Chevion M. Frontiers in Neurodegenerative Disorders and Aging: Fundamental Aspects, Clinical Perspectives and New Insights. NATO Science Series ed. IOS Press; Amsterdam: 2004.
-
- Manning MC, Chou DK, Murphy BM, Payne RW, Katayama DS. Stability of protein pharmaceuticals: an update. Pharm. Res. 2010;27(4):544–575. - PubMed
-
- Toennies G, Callan TP. Methionine studies: III. A comparison of oxidative reactions of methionine, cysteine, and cystine. Determination of methionine by hydrogen peroxide oxidation. J. Biol. Chem. 1939;129(2):481–490.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

