The sox family of transcription factors: versatile regulators of stem and progenitor cell fate
- PMID: 23290134
- PMCID: PMC3608206
- DOI: 10.1016/j.stem.2012.12.007
The sox family of transcription factors: versatile regulators of stem and progenitor cell fate
Abstract
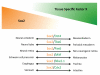
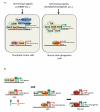
Sox family transcription factors are well-established regulators of cell fate decisions during development. Accumulating evidence documents that they play additional roles in adult tissue homeostasis and regeneration. Remarkably, forced expression of Sox factors, in combination with other synergistic factors, reprograms differentiated cells into somatic or pluripotent stem cells. Dysregulation of Sox factors has been further implicated in diseases including cancer. Here, we review molecular and functional evidence linking Sox proteins with stem cell biology, cellular reprogramming, and disease with an emphasis on Sox2.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


References
-
- Adameyko I, Lallemend F, Furlan A, Zinin N, Aranda S, Kitambi S, Blanchart A, Favaro R, Nicolis S, Lubke M, et al. Sox2 and Mitf cross-regulatory interactions consolidate progenitor and melanocyte lineages in the cranial neural crest. Development (Cambridge, England) 2012;139:397–807. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

