Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells
- PMID: 23290136
- PMCID: PMC6592822
- DOI: 10.1016/j.stem.2012.10.011
Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells
Abstract
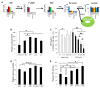
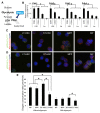
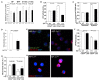
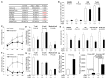
Defining the metabolic programs that underlie stem cell maintenance will be essential for developing strategies to manipulate stem cell capacity. Mammalian hematopoietic stem cells (HSCs) maintain cell cycle quiescence in a hypoxic microenvironment. It has been proposed that HSCs exhibit a distinct metabolic phenotype under these conditions. Here we directly investigated this idea using metabolomic analysis and found that HSCs generate adenosine-5'-triphosphate by anaerobic glycolysis through a pyruvate dehydrogenase kinase (Pdk)-dependent mechanism. Elevated Pdk expression leads to active suppression of the influx of glycolytic metabolites into mitochondria. Pdk overexpression in glycolysis-defective HSCs restored glycolysis, cell cycle quiescence, and stem cell capacity, while loss of both Pdk2 and Pdk4 attenuated HSC quiescence, glycolysis, and transplantation capacity. Moreover, treatment of HSCs with a Pdk mimetic promoted their survival and transplantation capacity. Thus, glycolytic metabolic status governed by Pdk acts as a cell cycle checkpoint that modulates HSC quiescence and function.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Stem cells: A metabolic switch.Nat Rev Mol Cell Biol. 2013 Feb;14(2):64-5. doi: 10.1038/nrm3515. Nat Rev Mol Cell Biol. 2013. PMID: 23340568 No abstract available.
References
-
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. - PubMed
-
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. - PubMed
-
- Dunford EC, Herbst EA, Jeoung NH, Gittings W, Inglis JG, Vandenboom R, LeBlanc PJ, Harris RA, Peters SJ. PDH activation during in vitro muscle contractions in PDH kinase 2 knockout mice: effect of PDH kinase 1 compensation. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1487–R1493. - PubMed
-
- Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

