A new platelet cryoprecipitate glue promoting bone formation after ectopic mesenchymal stromal cell-loaded biomaterial implantation in nude mice
- PMID: 23290259
- PMCID: PMC3706764
- DOI: 10.1186/scrt149
A new platelet cryoprecipitate glue promoting bone formation after ectopic mesenchymal stromal cell-loaded biomaterial implantation in nude mice
Abstract
Introduction: This study investigated the promising effect of a new Platelet Glue obtained from Cryoprecipitation of Apheresis Platelet products (PGCAP) used in combination with Mesenchymal Stromal Cells (MSC) loaded on ceramic biomaterials to provide novel strategies enhancing bone repair.
Methods: PGCAP growth factor content was analyzed by ELISA and compared to other platelet and plasma-derived products. MSC loaded on biomaterials (65% hydroxyapatite/35% beta-TCP or 100% beta-TCP) were embedded in PGCAP and grown in presence or not of osteogenic induction medium for 21 days. Biomaterials were then implanted subcutaneously in immunodeficient mice for 28 days. Effect of PGCAP on MSC was evaluated in vitro by proliferation and osteoblastic gene expression analysis and in vivo by histology and immunohistochemistry.
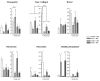
Results: We showed that PGCAP, compared to other platelet-derived products, allowed concentrating large amount of growth factors and cytokines which promoted MSC and osteoprogenitor proliferation. Next, we found that PGCAP improves the proliferation of MSC and osteogenic-induced MSC. Furthermore, we demonstrated that PGCAP up-regulates the mRNA expression of osteogenic markers (Collagen type I, Osteonectin, Osteopontin and Runx2). In vivo, type I collagen expressed in ectopic bone-like tissue was highly enhanced in biomaterials embedded in PGCAP in the absence of osteogenic pre-induction. Better results were obtained with 65% hydroxyapatite/35% beta-TCP biomaterials as compared to 100% beta-TCP.
Conclusions: We have demonstrated that PGCAP is able to enhance in vitro MSC proliferation, osteoblastic differentiation and in vivo bone formation in the absence of osteogenic pre-induction. This clinically adaptable platelet glue could be of interest for improving bone repair.
Figures
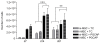

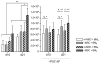



Similar articles
-
Influence of platelet-rich plasma on osteogenic differentiation of mesenchymal stem cells and ectopic bone formation in calcium phosphate ceramics.Cells Tissues Organs. 2006;183(2):68-79. doi: 10.1159/000095511. Cells Tissues Organs. 2006. PMID: 17053323
-
Platelet-rich plasma improves expansion of human mesenchymal stem cells and retains differentiation capacity and in vivo bone formation in calcium phosphate ceramics.Platelets. 2006 Nov;17(7):462-9. doi: 10.1080/09537100600758867. Platelets. 2006. PMID: 17074722
-
Ectopic bone formation associated with mesenchymal stem cells in a resorbable calcium deficient hydroxyapatite carrier.Biomaterials. 2005 Oct;26(29):5879-89. doi: 10.1016/j.biomaterials.2005.03.001. Biomaterials. 2005. PMID: 15913762
-
Immune Modulation by Transplanted Calcium Phosphate Biomaterials and Human Mesenchymal Stromal Cells in Bone Regeneration.Front Immunol. 2019 Apr 2;10:663. doi: 10.3389/fimmu.2019.00663. eCollection 2019. Front Immunol. 2019. PMID: 31001270 Free PMC article. Review.
-
Human cells with osteogenic potential in bone tissue research.Biomed Eng Online. 2023 Apr 3;22(1):33. doi: 10.1186/s12938-023-01096-w. Biomed Eng Online. 2023. PMID: 37013601 Free PMC article. Review.
Cited by
-
Engineering extracellular vesicles for ROS scavenging and tissue regeneration.Nano Converg. 2024 Jun 26;11(1):24. doi: 10.1186/s40580-024-00430-9. Nano Converg. 2024. PMID: 38922501 Free PMC article. Review.
-
The revolutionary role of placental derivatives in biomedical research.Bioact Mater. 2025 Mar 19;49:456-485. doi: 10.1016/j.bioactmat.2025.03.011. eCollection 2025 Jul. Bioact Mater. 2025. PMID: 40177109 Free PMC article. Review.
-
Ectopic Bone Tissue Engineering in Mice Using Human Gingiva or Bone Marrow-Derived Stromal/Progenitor Cells in Scaffold-Hydrogel Constructs.Front Bioeng Biotechnol. 2021 Nov 30;9:783468. doi: 10.3389/fbioe.2021.783468. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34917602 Free PMC article.
-
Bone regeneration in rat calvarial defects using dissociated or spheroid mesenchymal stromal cells in scaffold-hydrogel constructs.Stem Cell Res Ther. 2021 Nov 14;12(1):575. doi: 10.1186/s13287-021-02642-w. Stem Cell Res Ther. 2021. PMID: 34776000 Free PMC article.
-
Mesenchymal stem cells and their chondrogenic differentiated and dedifferentiated progeny express chemokine receptor CCR9 and chemotactically migrate toward CCL25 or serum.Stem Cell Res Ther. 2013 Aug 19;4(4):99. doi: 10.1186/scrt310. Stem Cell Res Ther. 2013. PMID: 23958031 Free PMC article.
References
-
- Heiple KG, Goldberg VM, Powell AE, Bos GD, Zika JM. Biology of cancellous bone grafts. Orthop Clin North Am. 1987;18:179–185. - PubMed
-
- Drosse I, Volkmer E, Capanna R, De Biase P, Mutschler W, Schieker M. Tissue engineering for bone defect healing: an update on a multi-component approach. Injury. 2008;39(Suppl 2):S9–20. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

