Targeting HER2-positive cancer cells with receptor-redirected anthrax protective antigen
- PMID: 23290417
- PMCID: PMC3621010
- DOI: 10.1016/j.molonc.2012.12.003
Targeting HER2-positive cancer cells with receptor-redirected anthrax protective antigen
Abstract
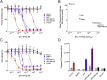
Targeted therapeutics have emerged in recent years as an attractive approach to treating various types of cancer. One approach is to modify a cytocidal protein toxin to direct its action to a specific population of cancer cells. We created a targeted toxin in which the receptor-binding and pore-forming moiety of anthrax toxin, termed Protective Antigen (PA), was modified to redirect its receptor specificity to HER2, a marker expressed at the surface of a significant fraction of breast and ovarian tumors. The resulting fusion protein (mPA-ZHER2) delivered cytocidal effectors specifically into HER2-positive tumor cells, including a trastuzumab-resistant line, causing death of the cells. No off-target killing of HER2-negative cells was observed, either with homogeneous populations or with mixtures of HER2-positive and HER2-negative cells. A mixture of mPA variants targeting different receptors mediated killing of cells bearing either receptor, without affecting cells devoid of these receptors. Anthrax toxin may serve as an effective platform for developing therapeutics to ablate cells bearing HER2 or other tumor-specific cell-surface markers.
Copyright © 2012 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Changing the receptor specificity of anthrax toxin.mBio. 2012 May 1;3(3):e00088-12. doi: 10.1128/mBio.00088-12. Print 2012. mBio. 2012. PMID: 22550037 Free PMC article.
-
Targeted delivery of an ADP-ribosylating bacterial toxin into cancer cells.Sci Rep. 2017 Jan 27;7:41252. doi: 10.1038/srep41252. Sci Rep. 2017. PMID: 28128281 Free PMC article.
-
Affibody-derived drug conjugates: Potent cytotoxic molecules for treatment of HER2 over-expressing tumors.J Control Release. 2018 Oct 28;288:84-95. doi: 10.1016/j.jconrel.2018.08.040. Epub 2018 Aug 30. J Control Release. 2018. PMID: 30172673
-
Tumor Targeting and Drug Delivery by Anthrax Toxin.Toxins (Basel). 2016 Jul 1;8(7):197. doi: 10.3390/toxins8070197. Toxins (Basel). 2016. PMID: 27376328 Free PMC article. Review.
-
Novel HER2-Targeting Antibody-Drug Conjugates of Trastuzumab Beyond T-DM1 in Breast Cancer: Trastuzumab Deruxtecan(DS-8201a) and (Vic-)Trastuzumab Duocarmazine (SYD985).Eur J Med Chem. 2019 Dec 1;183:111682. doi: 10.1016/j.ejmech.2019.111682. Epub 2019 Sep 6. Eur J Med Chem. 2019. PMID: 31563805 Review.
Cited by
-
Combination of Near-Infrared Photoimmunotherapy Using Trastuzumab and Small Protein Mimetic for HER2-Positive Breast Cancer.Int J Mol Sci. 2021 Nov 11;22(22):12213. doi: 10.3390/ijms222212213. Int J Mol Sci. 2021. PMID: 34830099 Free PMC article.
-
Reengineering anthrax toxin protective antigen for improved receptor-specific protein delivery.BMC Biol. 2020 Aug 13;18(1):100. doi: 10.1186/s12915-020-00827-y. BMC Biol. 2020. PMID: 32792013 Free PMC article.
-
Bacillus anthracis' PA63 Delivers the Tumor Metastasis Suppressor Protein NDPK-A/NME1 into Breast Cancer Cells.Int J Mol Sci. 2020 May 6;21(9):3295. doi: 10.3390/ijms21093295. Int J Mol Sci. 2020. PMID: 32384736 Free PMC article.
-
Prodrug applications for targeted cancer therapy.AAPS J. 2014 Sep;16(5):899-913. doi: 10.1208/s12248-014-9638-z. Epub 2014 Jul 9. AAPS J. 2014. PMID: 25004822 Free PMC article. Review.
-
A bacterial toxin that cleaves Ras oncoprotein.Oncotarget. 2015 Aug 7;6(22):18742-3. doi: 10.18632/oncotarget.5113. Oncotarget. 2015. PMID: 26300052 Free PMC article. No abstract available.
References
-
- Abi-Habib, R.J. , 2006. A urokinase-activated recombinant anthrax toxin is selectively cytotoxic to many human tumor cell types. Molecular Cancer Therapeutics 5, (10) 2556–2562. - PubMed
-
- Arteaga, C.L. , 2012. Treatment of HER2-positive breast cancer: current status and future perspectives. Nature Reviews Clinical Oncology 9, (1) 16–32. - PubMed
-
- Berchuck, A. , 1990. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Research 50, (13) 4087–4091. - PubMed
-
- Bradley, K.A. , 2001. Identification of the cellular receptor for anthrax toxin. Nature 414, (6860) 225–229. - PubMed
-
- Carter, P.J. , Senter, P.D. , 2008. Antibody-drug conjugates for cancer therapy. Cancer Journal (Sudbury, Mass.) 14, (3) 154–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

