Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study
- PMID: 23290630
- PMCID: PMC3563251
- DOI: 10.1016/S1474-4422(12)70310-1
Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study
Abstract
Background: Anti-NMDA receptor (NMDAR) encephalitis is an autoimmune disorder in which the use of immunotherapy and the long-term outcome have not been defined. We aimed to assess the presentation of the disease, the spectrum of symptoms, immunotherapies used, timing of improvement, and long-term outcome.
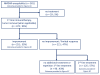
Methods: In this multi-institutional observational study, we tested for the presence of NMDAR antibodies in serum or CSF samples of patients with encephalitis between Jan 1, 2007, and Jan 1, 2012. All patients who tested positive for NMDAR antibodies were included in the study; patients were assessed at symptom onset and at months 4, 8, 12, 18, and 24, by use of the modified Rankin scale (mRS). Treatment included first-line immunotherapy (steroids, intravenous immunoglobulin, plasmapheresis), second-line immunotherapy (rituximab, cyclophosphamide), and tumour removal. Predictors of outcome were determined at the Universities of Pennsylvania (PA, USA) and Barcelona (Spain) by use of a generalised linear mixed model with binary distribution.
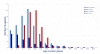
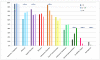
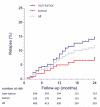
Results: We enrolled 577 patients (median age 21 years, range 8 months to 85 years), 211 of whom were children (<18 years). Treatment effects and outcome were assessable in 501 (median follow-up 24 months, range 4-186): 472 (94%) underwent first-line immunotherapy or tumour removal, resulting in improvement within 4 weeks in 251 (53%). Of 221 patients who did not improve with first-line treatment, 125 (57%) received second-line immunotherapy that resulted in a better outcome (mRS 0-2) than those who did not (odds ratio [OR] 2·69, CI 1·24-5·80; p=0·012). During the first 24 months, 394 of 501 patients achieved a good outcome (mRS 0-2; median 6 months, IQR 2-12) and 30 died. At 24 months' follow-up, 203 (81%) of 252 patients had good outcome. Outcomes continued to improve for up to 18 months after symptom onset. Predictors of good outcome were early treatment (0·62, 0·50-0·76; p<0·0001) and no admission to an intensive care unit (0·12, 0·06-0·22; p<0·0001). 45 patients had one or multiple relapses (representing a 12% risk within 2 years); 46 (67%) of 69 relapses were less severe than initial episodes (p<0·0001). In 177 children, predictors of good outcome and the magnitude of effect of second-line immunotherapy were similar to those of the entire cohort.
Interpretation: Most patients with anti-NMDAR encephalitis respond to immunotherapy. Second-line immunotherapy is usually effective when first-line treatments fail. In this cohort, the recovery of some patients took up to 18 months.
Funding: The Dutch Cancer Society, the National Institutes of Health, the McKnight Neuroscience of Brain Disorders award, The Fondo de Investigaciones Sanitarias, and Fundació la Marató de TV3.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures








Comment in
-
The importance of early and sustained treatment of a common autoimmune encephalitis.Lancet Neurol. 2013 Feb;12(2):123-5. doi: 10.1016/S1474-4422(12)70319-8. Epub 2013 Jan 3. Lancet Neurol. 2013. PMID: 23290629 No abstract available.
-
Antibody mediated encephalitis.J Neurol. 2013 Apr;260(4):1187-90. doi: 10.1007/s00415-013-6890-6. J Neurol. 2013. PMID: 23515700 No abstract available.
-
Prevalence and treatment of anti-NMDA receptor encephalitis.Lancet Neurol. 2013 May;12(5):424. doi: 10.1016/S1474-4422(13)70069-3. Lancet Neurol. 2013. PMID: 23602155 No abstract available.
-
Prevalence and treatment of anti-NMDA receptor encephalitis.Lancet Neurol. 2013 May;12(5):424-5. doi: 10.1016/S1474-4422(13)70070-X. Lancet Neurol. 2013. PMID: 23602156 No abstract available.
-
Authors' reply.Lancet Neurol. 2013 May;12(5):425-6. doi: 10.1016/S1474-4422(13)70072-3. Lancet Neurol. 2013. PMID: 23602157 No abstract available.
References
-
- Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

