Urinary chemokine (C-C motif) ligand 2 (monocyte chemotactic protein-1) as a tubular injury marker for early detection of cisplatin-induced nephrotoxicity
- PMID: 23291264
- PMCID: PMC4077184
- DOI: 10.1016/j.bcp.2012.12.019
Urinary chemokine (C-C motif) ligand 2 (monocyte chemotactic protein-1) as a tubular injury marker for early detection of cisplatin-induced nephrotoxicity
Abstract
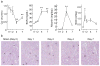
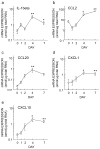
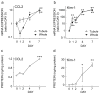
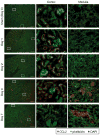
Because of the difficulty in detecting segment-specific response in the kidney, we investigated the molecular events underlying acute kidney injury in the proximal tubules of rats with cisplatin (cis-diamminedichloroplatinum II)-induced nephrotoxicity. Microarray analysis revealed that mRNA levels of several cytokines and chemokines, such as interleukin-1beta, chemokine (C-C motif) ligand (CCL) 2, CCL20, chemokine (C-X-C motif) ligand (CXCL) 1, and CXCL10 were significantly increased after cisplatin treatment in both isolated proximal tubules and whole kidney. Interestingly, tubular CCL2 mRNA levels increased soon after cisplatin administration, whereas CCL2 mRNA levels in whole kidney first decreased and then increased. Levels of both CCL2 and kidney injury molecule-1 (KIM-1) in the whole kidney increased after cisplatin administration. Immunofluorescence analysis revealed CCL2 changes in the proximal tubular cells initially and then in the medullary interstitium. Urinary CCL2 excretion significantly increased approximately 3-fold compared with controls the day after cisplatin administration (5mg/kg), when no changes were observed plasma creatinine and blood urea nitrogen levels. Urinary levels of KIM-1 also increased 3-fold after cisplatin administration. In addition, urinary CCL2 rather than KIM-1 increased in chronic renal failure rats after administration of low-dose cisplatin (2mg/kg), suggesting that urinary CCL2 was selective for cisplatin-induced nephrotoxicity in renal impairment. These results indicated that the increase in cytokine and chemokine expression in renal epithelial cells might be responsible for kidney deterioration in cisplatin-induced nephrotoxicity, and that urinary CCL2 is associated with tubular injury and serves as a sensitive and noninvasive marker for the early detection of cisplatin-induced tubular injury.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




References
-
- Perazella MA. Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol. 2009;4:1275–83. - PubMed
-
- Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613–21. - PubMed
-
- Inui K, Masuda S, Saito H. Cellular and molecular aspects of drug transport in the kidney. Kidney Int. 2000;58:944–58. - PubMed
-
- Yonezawa A, Masuda S, Nishihara K, Yano I, Katsura T, Inui K. Association between tubular toxicity of cisplatin and expression of organic cation transporter rOCT2 (Slc22a2) in the rat. Biochem Pharmacol. 2005;70:1823–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

