Confessions of an icosahedral virus crystallographer
- PMID: 23291268
- PMCID: PMC3624677
- DOI: 10.1093/jmicro/dfs097
Confessions of an icosahedral virus crystallographer
Abstract
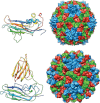
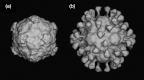
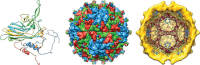
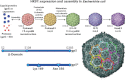
This is a personal history of my structural studies of icosahedral viruses that evolved from crystallographic studies, to hybrid methods with electron cryo-microscopy and image reconstruction (cryoEM) and then developed further by incorporating a variety of physical methods to augment the high resolution crystallographic studies. It is not meant to be comprehensive, even for my own work, but hopefully provides some perspective on the growth of our understanding of these remarkable biologic assemblies. The goal is to provide a historical perspective for those new to the field and to emphasize the limitations of any one method, even those that provide atomic resolution information about viruses.
Figures

References
-
- Harrison S C. A point-focusing camera for single-crystal diffraction. J. Appl. Cryst. 1968;1:84–90. doi:10.1107/S0021889868005054. - DOI
-
- Harrison S C. Structure of tomato bushy stunt virus. I. The spherically averaged electron density. J. Mol. Biol. 1969;42:457–483. doi:10.1016/0022-2836(69)90236-8. - DOI - PubMed
-
- Harrison S C. Structure of tomato bushy stunt virus at 25 Å resolution. Cold Spring Harb. Symp. Quant. Biol. 1971;36:495–501. doi:10.1101/SQB.1972.036.01.063. - DOI - PubMed
-
- Harrison S C, Jack A. Structure of tomato bushy stunt virus. Three-dimensional x-ray diffraction analysis at 16 A resolution. J. Mol. Biol. 1975;97:173–191. doi:10.1016/S0022-2836(75)80033-7. - DOI - PubMed
-
- Jack A, Harrison S C, Crowther R A. Structure of tomato bushy stunt virus. II. Comparison of results obtained by electron microscopy and x-ray diffraction. J. Mol. Biol. 1975;97:163–172. doi:10.1016/S0022-2836(75)80032-5. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

