Changes in hypothalamic expression of the Lin28/let-7 system and related microRNAs during postnatal maturation and after experimental manipulations of puberty
- PMID: 23291449
- PMCID: PMC3548186
- DOI: 10.1210/en.2012-2006
Changes in hypothalamic expression of the Lin28/let-7 system and related microRNAs during postnatal maturation and after experimental manipulations of puberty
Abstract
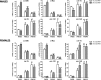
Lin28 and Lin28b are related RNA-binding proteins that inhibit the maturation of miRNAs of the let-7 family and participate in the control of cellular stemness and early embryonic development. Considerable interest has arisen recently concerning other physiological roles of the Lin28/let-7 axis, including its potential involvement in the control of puberty, as suggested by genome-wide association studies and functional genomics. We report herein the expression profiles of Lin28 and let-7 members in the rat hypothalamus during postnatal maturation and in selected models of altered puberty. The expression patterns of c-Myc (upstream positive regulator of Lin28), mir-145 (negative regulator of c-Myc), and mir-132 and mir-9 (putative miRNA repressors of Lin28, predicted by bioinformatic algorithms) were also explored. In male and female rats, Lin28, Lin28b, and c-Myc mRNAs displayed very high hypothalamic expression during the neonatal period, markedly decreased during the infantile-to-juvenile transition and reached minimal levels before/around puberty. A similar puberty-related decline was observed for Lin28b in monkey hypothalamus but not in the rat cortex, suggesting species conservation and tissue specificity. Conversely, let-7a, let-7b, mir-132, and mir-145, but not mir-9, showed opposite expression profiles. Perturbation of brain sex differentiation and puberty, by neonatal treatment with estrogen or androgen, altered the expression ratios of Lin28/let-7 at the time of puberty. Changes in the c-Myc/Lin28b/let-7 pathway were also detected in models of delayed puberty linked to early photoperiod manipulation and, to a lesser extent, postnatal underfeeding or chronic subnutrition. Altogether, our data are the first to document dramatic changes in the expression of the Lin28/let-7 axis in the rat hypothalamus during the postnatal maturation and after different manipulations that disturb puberty, thus suggesting the potential involvement of developmental changes in hypothalamic Lin28/let-7 expression in the mechanisms permitting/leading to puberty onset.
Figures





References
-
- Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416 - PubMed
-
- Moss EG, Tang L. Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev Biol. 2003;258:432–442 - PubMed
-
- Balzer E, Moss EG. Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA Biol. 2007;4:16–25 - PubMed
-
- Guo Y, Chen Y, Ito H, Watanabe A, Ge X, Kodama T, Aburatani H. Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene. 2006;384:51–61 - PubMed
-
- Pasquinelli AE, McCoy A, Jimenez E, Salo E, Ruvkun G, Martindale MQ, Baguna J. Expression of the 22 nucleotide let-7 heterochronic RNA throughout the Metazoa: a role in life history evolution? Evol Dev. 2003;5:372–378 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

