Primary human chondrocyte extracellular matrix formation and phenotype maintenance using RGD-derivatized PEGDM hydrogels possessing a continuous Young's modulus gradient
- PMID: 23291491
- PMCID: PMC3799765
- DOI: 10.1016/j.actbio.2012.12.028
Primary human chondrocyte extracellular matrix formation and phenotype maintenance using RGD-derivatized PEGDM hydrogels possessing a continuous Young's modulus gradient
Abstract
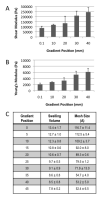
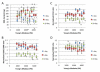
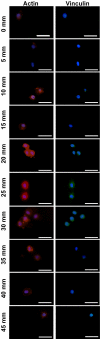
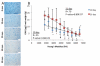
Efficient ex vivo methods for expanding primary human chondrocytes while maintaining the phenotype is critical to advancing the sourcing of autologous cells for tissue engineering applications. While there has been significant research reported in the literature, systematic approaches are necessary to determine and optimize the chemical and mechanical scaffold properties for hyaline cartilage generation using limited cell numbers. Functionalized hydrogels possessing continuous variations in physico-chemical properties are, therefore, an efficient three-dimensional platform for studying several properties simultaneously. Herein we describe a polyethylene glycol dimethacrylate (PEGDM) hydrogel system with a modulus gradient (~27,000-3800 Pa) containing a uniform concentration of arginine-glycine-aspartic acid (RGD) peptide to enhance cell adhesion in order to correlate primary human osteoarthritic chondrocyte proliferation, phenotype maintenance, and extracellular matrix (ECM) production with hydrogel properties. Cell number and chondrogenic phenotype (CD14:CD90 ratios) were found to decline in regions with a higher storage modulus (>13,100 Pa), while regions with a lower storage modulus maintained their cell number and phenotype. Over 3 weeks culture hydrogel regions possessing a lower Young's modulus experienced an increase in ECM content (~200%) compared with regions with a higher storage modulus. Variations in the amount and organization of the cytoskeletal markers actin and vinculin were observed within the modulus gradient, which are indicative of differences in chondrogenic phenotype maintenance and ECM expression. Thus scaffold mechanical properties have a significant impact in modulating human osteoarthritic chondrocyte behavior and tissue formation.
Copyright © 2012 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures



References
-
- Lane NE, Brandt K, Hawker G, Peeva E, Schreyer E, Tsuji W, et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis and Cartilage. 2011;19:478–82. - PubMed
-
- Squires GR, Okouneff S, Ionescu M, Poole AR. The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis. Arthritis & Rheumatism. 2003;48:1261–70. - PubMed
-
- Lorenz H, Richter W. Osteoarthritis: Cellular and molecular changes in degenerating cartilage. Progress in Histochemistry and Cytochemistry. 2006;40:135–63. - PubMed
-
- Garnero P, Ayral X, Rousseau J-C, Christgau S, Sandell LJ, Dougados M, et al. Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis & Rheumatism. 2002;46:2613–24. - PubMed
-
- Simon CG, Lin-Gibson S. Combinatorial and High-Throughput Screening of Biomaterials. Advanced Materials. 2011;23:369–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

