MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8⁺ T cells
- PMID: 23291597
- PMCID: PMC3581685
- DOI: 10.1038/ni.2513
MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8⁺ T cells
Abstract
Myelin presentation to T cells in the central nervous system (CNS) sustains inflammation in multiple sclerosis (MS). CD4(+) and CD8(+) T cells contribute to MS, but only cells that present myelin to CD4(+) T cells have been identified. We show that MHC class I-restricted myelin basic protein (MBP) was presented by oligodendrocytes and cross-presented by Tip-dendritic cells (DCs) during experimental autoimmune encephalomyelitis (EAE), an animal model of MS initiated by CD4(+) T cells. Tip-DCs activated naive and effector CD8(+) T cells ex vivo, and naive MBP-specific CD8(+) T cells were activated in the CNS during CD4(+) T cell-induced EAE. These results demonstrate that CD4(+) T cell-mediated CNS autoimmunity leads to determinant spreading to myelin-specific CD8(+) T cells that can directly recognize oligodendrocytes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



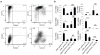

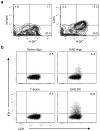
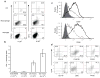
Comment in
-
A Tip leads cytotoxic T cells to the crime scene in neuroinflammation.Nat Immunol. 2013 Mar;14(3):196-7. doi: 10.1038/ni.2551. Nat Immunol. 2013. PMID: 23416668 No abstract available.
References
-
- Tompkins SM, et al. De novo central nervous system processing of myelin antigen is required for the initiation of experimental autoimmune encephalomyelitis. J Immunol. 2002;168:4173–4183. - PubMed
-
- Greter M, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

