Cyclin E controls Drosophila female germline stem cell maintenance independently of its role in proliferation by modulating responsiveness to niche signals
- PMID: 23293285
- PMCID: PMC3561789
- DOI: 10.1242/dev.088583
Cyclin E controls Drosophila female germline stem cell maintenance independently of its role in proliferation by modulating responsiveness to niche signals
Abstract
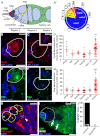
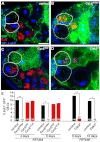
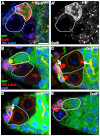
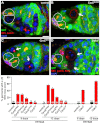
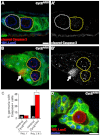
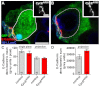
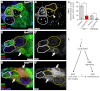
Stem cells must proliferate while maintaining 'stemness'; however, much remains to be learned about how factors that control the division of stem cells influence their identity. Multiple stem cell types display cell cycles with short G1 phases, thought to minimize susceptibility to differentiation factors. Drosophila female germline stem cells (GSCs) have short G1 and long G2 phases, and diet-dependent systemic factors often modulate G2. We previously observed that Cyclin E (CycE), a known G1/S regulator, is atypically expressed in GSCs during G2/M; however, it remained unclear whether CycE has cell cycle-independent roles in GSCs or whether it acts exclusively by modulating the cell cycle. In this study, we detected CycE activity during G2/M, reflecting its altered expression pattern, and showed that CycE and its canonical partner, Cyclin-dependent kinase 2 (Cdk2), are required not only for GSC proliferation, but also for GSC maintenance. In genetic mosaics, CycE- and Cdk2-deficient GSCs are rapidly lost from the niche, remain arrested in a G1-like state, and undergo excessive growth and incomplete differentiation. However, we found that CycE controls GSC maintenance independently of its role in the cell cycle; GSCs harboring specific hypomorphic CycE mutations are not efficiently maintained despite normal proliferation rates. Finally, CycE-deficient GSCs have an impaired response to niche bone morphogenetic protein signals that are required for GSC self-renewal, suggesting that CycE modulates niche-GSC communication. Taken together, these results show unequivocally that the roles of CycE/Cdk2 in GSC division cycle regulation and GSC maintenance are separable, and thus potentially involve distinct sets of phosphorylation targets.
Figures
References
-
- Ashburner M., Drysdale R. (1994). FlyBase – the Drosophila genetic database. Development 120, 2077-2079 - PubMed
-
- Berger C., Pallavi S. K., Prasad M., Shashidhara L. S., Technau G. M. (2005). A critical role for cyclin E in cell fate determination in the central nervous system of Drosophila melanogaster. Nat. Cell Biol. 7, 56-62 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

