Lrp4 and Wise interplay controls the formation and patterning of mammary and other skin appendage placodes by modulating Wnt signaling
- PMID: 23293290
- PMCID: PMC6514302
- DOI: 10.1242/dev.085118
Lrp4 and Wise interplay controls the formation and patterning of mammary and other skin appendage placodes by modulating Wnt signaling
Abstract
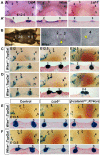
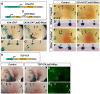
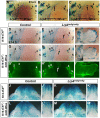
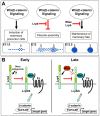
The future site of skin appendage development is marked by a placode during embryogenesis. Although Wnt/β-catenin signaling is known to be essential for skin appendage development, it is unclear which cellular processes are controlled by the signaling and how the precise level of the signaling activity is achieved during placode formation. We have investigated roles for Lrp4 and its potential ligand Wise (Sostdc1) in mammary and other skin appendage placodes. Lrp4 mutant mice displayed a delay in placode initiation and changes in distribution and number of mammary precursor cells leading to abnormal morphology, number and position of mammary placodes. These Lrp4 mammary defects, as well as limb defects, were associated with elevated Wnt/β-catenin signaling and were rescued by reducing the dose of the Wnt co-receptor genes Lrp5 and Lrp6, or by inactivating the gene encoding β-catenin. Wise-null mice phenocopied a subset of the Lrp4 mammary defects and Wise overexpression reduced the number of mammary precursor cells. Genetic epistasis analyses suggest that Wise requires Lrp4 to exert its function and that, together, they have a role in limiting mammary fate, but Lrp4 has an early Wise-independent role in facilitating placode formation. Lrp4 and Wise mutants also share defects in vibrissa and hair follicle development, suggesting that the roles played by Lrp4 and Wise are common to skin appendages. Our study presents genetic evidence for interplay between Lrp4 and Wise in inhibiting Wnt/β-catenin signaling and provides an insight into how modulation of Wnt/β-catenin signaling controls cellular processes important for skin placode formation.
Figures





References
-
- Balemans W., Ebeling M., Patel N., Van Hul E., Olson P., Dioszegi M., Lacza C., Wuyts W., Van Den Ende J., Willems P., et al. (2001). Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 10, 537-543. - PubMed
-
- Boras-Granic K., Chang H., Grosschedl R., Hamel P. A. (2006). Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Dev. Biol. 295, 219-231. - PubMed
-
- Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D. H., McMahon A. P., Sommer L., Boussadia O., Kemler R. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253-1264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

