Sox17 haploinsufficiency results in perinatal biliary atresia and hepatitis in C57BL/6 background mice
- PMID: 23293295
- PMCID: PMC3561787
- DOI: 10.1242/dev.086702
Sox17 haploinsufficiency results in perinatal biliary atresia and hepatitis in C57BL/6 background mice
Abstract
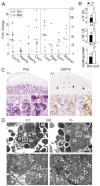
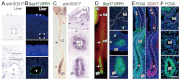
Congenital biliary atresia is an incurable disease of newborn infants, of unknown genetic causes, that results in congenital deformation of the gallbladder and biliary duct system. Here, we show that during mouse organogenesis, insufficient SOX17 expression in the gallbladder and bile duct epithelia results in congenital biliary atresia and subsequent acute 'embryonic hepatitis', leading to perinatal death in ~95% of the Sox17 heterozygote neonates in C57BL/6 (B6) background mice. During gallbladder and bile duct development, Sox17 was expressed at the distal edge of the gallbladder primordium. In the Sox17(+/-) B6 embryos, gallbladder epithelia were hypoplastic, and some were detached from the luminal wall, leading to bile duct stenosis or atresia. The shredding of the gallbladder epithelia is probably caused by cell-autonomous defects in proliferation and maintenance of the Sox17(+/-) gallbladder/bile duct epithelia. Our results suggest that Sox17 plays a dosage-dependent function in the morphogenesis and maturation of gallbladder and bile duct epithelia during the late-organogenic stages, highlighting a novel entry point to the understanding of the etiology and pathogenesis of human congenital biliary atresia.
Figures





References
-
- Bamford R. N., Roessler E., Burdine R. D., Saplako lu U., dela Cruz J., Splitt M., Goodship J. A., Towbin J., Bowers P., Ferrero G. B., et al. (2000). Loss-of-function mutations in the EGF-CFC gene CFC1 are associated with human left-right laterality defects. Nat. Genet. 26, 365-369 - PubMed
-
- Bernstein H., Payne C. M., Bernstein C., Schneider J., Beard S. E., Crowley C. L. (1999). Activation of the promoters of genes associated with DNA damage, oxidative stress, ER stress and protein malfolding by the bile salt, deoxycholate. Toxicol. Lett. 108, 37-46 - PubMed
-
- Cardinale V., Wang Y., Carpino G., Cui C. B., Gatto M., Rossi M., Berloco P. B., Cantafora A., Wauthier E., Furth M. E., et al. (2011). Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology 54, 2159-2172 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

