HLA class II molecules influence susceptibility versus protection in inflammatory diseases by determining the cytokine profile
- PMID: 23293357
- PMCID: PMC3545203
- DOI: 10.4049/jimmunol.1201891
HLA class II molecules influence susceptibility versus protection in inflammatory diseases by determining the cytokine profile
Abstract
The MHC in humans encodes the most polymorphic genes, the HLA genes, which are critical for the immune system to clear infection. This can be attributed to strong selection pressure as populations moved to different parts of the world and encountered new kinds of infections, leading to new HLA class II alleles. HLA genes also have the highest relative risk for autoimmune diseases. Three haplotypes, that is, HLA-DR2DQ6, DR4DQ8, and DR3DQ2, account for HLA association with most autoimmune diseases. We hypothesize that these haplotypes, along with their multiple subtypes, have survived bottlenecks of infectious episodes in human history because of their ability to present pathogenic peptides to activate T cells that secrete cytokines to clear infections. Unfortunately, they also present self-peptides/mimics to activate autoreactive T cells secreting proinflammatory cytokines that cause autoimmune diseases.
Figures

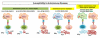
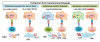
References
-
- Janeway CA, Travers P, Walport M, Shlomchik M. Immunobiology Garland Science. New York, NY: 2002.
-
- Klein J. Natural History of the Major Histocompatibility Complex. John Wiley & Sons; New York, NY, USA: 1986.
-
- Piertney SB, Oliver MK. The evolutionary ecology of the major histocompatibility complex. Heredity (Edinb) 2006;96:7–21. - PubMed
-
- Trowsdale J. The MHC, disease and selection. Immunol Lett. 2011;137:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

