Self-administration of subcutaneous depot medroxyprogesterone acetate by adolescent women
- PMID: 23294549
- PMCID: PMC3745808
- DOI: 10.1016/j.contraception.2012.11.019
Self-administration of subcutaneous depot medroxyprogesterone acetate by adolescent women
Abstract
Background: Intramuscular depot medroxyprogesterone acetate (DMPA-IM) is now available in subcutaneous (SC) formulation, potentially allowing for home-based self-administration. We examined adolescents' interest in and proficiency at DMPA-SC self-administration.
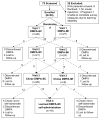
Study design: This is a planned secondary analysis of a randomized controlled trial comparing pain between DMPA-IM and DMPA-SC. In the trial, study participants (N=55) aged 14-21 years were recruited at DMPA initiation and randomized to receive DMPA-IM or DMPA-SC. Participants received the alternate formulation at 3 months, chose formulation at 6 months and could learn self-administration at 9 months. The current analysis is of the women who chose self-administration of DMPA-SC. Proficiency was rated for each step of self-administration: independently [I], with reassurance [R], with verbal instruction [V] or nurse performed [RN]. Data were analyzed using descriptive and comparative statistics.
Results: Thirty-five percent (19/55) of participants learned self-administration. Proficiency ratings were as follows: chose injection site (I=78.9%, R=5.3%, V=5.3%, RN=10.5%), cleaned site (I=89.5%, RN=10.5%), assembled injection device (I=47.4%, R=36.8%, V=15.8%), self-injected (I=31.6%, R=36.8%, V=15.8%, RN=15.8%) and disposed of device (I=21.1%, R=21.1%, RN=57.9%).
Conclusions: Many adolescents are interested in and capable of DMPA-SC self-administration with brief education and minimal assistance.
Keywords: Adolescent; Contraception; Depot medroxyprogesterone acetate; Self-injection.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- Martinez G, Copen CE, Abma JC. Teenagers in the United States: Sexual activity, contraceptive use, and childbearing, 2006–2010 National Survey of Family Growth. National Center for Health Statistics. Vital Health Stat. 2011;23(31) - PubMed
-
- Abma JC, Martinez GM, Mosher WD, Dawson BS. Teenagers in the United States: Sexual activity, contraceptive use, and childbearing, 2002 National Survey of Family Growth. National Center for Health Statistics. Vital Health Stat. 2004;23(24) - PubMed
-
- Depo-SubQ Provera 104 [package insert] Pharmacia and Upjohn Co Division of Pfizer Inc; New York, NY: Oct, 2007. [Accessed June 1, 2012]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/021583lbl.pdf.
-
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269–75. - PubMed
-
- Jain J, Dutton C, Nicosia A, Wajszczuk C, Bode FR, Mishell DR. Pharmacokinetics, ovulation suppression and return to ovulation following a lower dose subcutaneous formulation of Depo-Provera. Contraception. 2004;70:11–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical