Comorbidities in ADHD children treated with methylphenidate: a database study
- PMID: 23294623
- PMCID: PMC3544568
- DOI: 10.1186/1471-244X-13-11
Comorbidities in ADHD children treated with methylphenidate: a database study
Abstract
Background: Methylphenidate (MPH) is the most common drug treatment of attention deficit / hyperactivity disorder (ADHD) in children. Treatment with MPH is contraindicated in the presence of certain psychiatric, cerebro- and cardiovascular conditions. We assessed MPH treatment prevalence and incidence and the frequency of comorbid conditions related to these contraindications in new MPH users compared to a control group without ADHD and ADHD medication.
Methods: We used health care data for the years 2004 to 2006 from the German Pharmacoepidemiological Research Database (GePaRD) which includes about 18% of the German population. MPH treatment prevalence and incidence was assessed based on at least one MPH prescription in the given year. In MPH users, the prevalence of psychiatric and other comorbidities was assessed in the quarter of the first MPH prescription and the three preceding quarters, whereas in controls it was assessed in the earliest four quarters of continuous insurance time starting at 01.01.2004 or the start of insurance if this was later. Differences in the presence of comorbid diagnoses between MPH users and controls were tested by logistic regression.
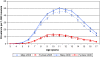
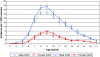
Results: In 2005, 1.5% of all children and adolescents aged 3 to 17 years (2.3% of males and 0.6% of females) received MPH in Germany. The proportion of children with a record of a psychiatric comorbidity in any of the nine ICD categories of diagnoses was substantially higher in new MPH users (83%) compared to controls (20%). Cerebro- and cardiovascular comorbidities were rare in general. Still, among new MPH users, 2% of males and females had a diagnosis of a pre-existing cardiovascular disorder but only 1.2% of controls.
Conclusions: Besides MPH treatment prevalence we first publish age-specific incidence rates for Germany. A high proportion of children who were started on MPH had a record of a psychiatric comorbidity preceding the first prescription. Cerebro- and cardiovascular conditions were rare in the studied age range, but still higher among children who received MPH than in the control group. Results show that in a substantial subgroup of patients, comorbidities require a thorough weighting of possible risks of MPH medication against the risks of untreated ADHD.
Figures
References
-
- Rappley MD. Safety issues in the use of methylphenidate. An American perspective. Drug Saf. 1997;17:143–148. - PubMed
-
- Schubert I, Koster I, Lehmkuhl G. The changing prevalence of attention-deficit/hyperactivity disorder and methylphenidate prescriptions: a study of data from a random sample of insurees of the AOK Health Insurance Company in the German State of Hesse, 2000–2007. Dtsch Arztebl Int. 2010;107:615–621. - PMC - PubMed
-
- Sullivan SS. Narcolepsy in adolescents. Adolesc Med State Art Rev. 2010;21:542–555. - PubMed
-
- Schwabe U, Paffrath D. Arzneiverordnungs-Report '91 Akutelle Daten, Kosten, Trends und Kommentare. Stuttgart, New York: Gustav Fischer Verlag; 1990.
-
- Schwabe U, Paffrath D. Arzneiverordnungs-Report 2010 Aktuelle Daten, Kosten, Trends und Kommentare. Berlin, Heidelberg: Springer; 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

