Multi-kernel graph embedding for detection, Gleason grading of prostate cancer via MRI/MRS
- PMID: 23294985
- PMCID: PMC3708492
- DOI: 10.1016/j.media.2012.10.004
Multi-kernel graph embedding for detection, Gleason grading of prostate cancer via MRI/MRS
Abstract
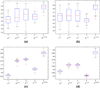
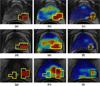
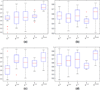
Even though 1 in 6 men in the US, in their lifetime are expected to be diagnosed with prostate cancer (CaP), only 1 in 37 is expected to die on account of it. Consequently, among many men diagnosed with CaP, there has been a recent trend to resort to active surveillance (wait and watch) if diagnosed with a lower Gleason score on biopsy, as opposed to seeking immediate treatment. Some researchers have recently identified imaging markers for low and high grade CaP on multi-parametric (MP) magnetic resonance (MR) imaging (such as T2 weighted MR imaging (T2w MRI) and MR spectroscopy (MRS)). In this paper, we present a novel computerized decision support system (DSS), called Semi Supervised Multi Kernel Graph Embedding (SeSMiK-GE), that quantitatively combines structural, and metabolic imaging data for distinguishing (a) benign versus cancerous, and (b) high- versus low-Gleason grade CaP regions from in vivo MP-MRI. A total of 29 1.5Tesla endorectal pre-operative in vivo MP MRI (T2w MRI, MRS) studies from patients undergoing radical prostatectomy were considered in this study. Ground truth for evaluation of the SeSMiK-GE classifier was obtained via annotation of disease extent on the pre-operative imaging by visually correlating the MRI to the ex vivo whole mount histologic specimens. The SeSMiK-GE framework comprises of three main modules: (1) multi-kernel learning, (2) semi-supervised learning, and (3) dimensionality reduction, which are leveraged for the construction of an integrated low dimensional representation of the different imaging and non-imaging MRI protocols. Hierarchical classifiers for diagnosis and Gleason grading of CaP are then constructed within this unified low dimensional representation. Step 1 of the hierarchical classifier employs a random forest classifier in conjunction with the SeSMiK-GE based data representation and a probabilistic pairwise Markov Random Field algorithm (which allows for imposition of local spatial constraints) to yield a voxel based classification of CaP presence. The CaP region of interest identified in Step 1 is then subsequently classified as either high or low Gleason grade CaP in Step 2. Comparing SeSMiK-GE with unimodal T2w MRI, MRS classifiers and a commonly used feature concatenation (COD) strategy, yielded areas (AUC) under the receiver operative curve (ROC) of (a) 0.89±0.09 (SeSMiK), 0.54±0.18 (T2w MRI), 0.61±0.20 (MRS), and 0.64±0.23 (COD) for distinguishing benign from CaP regions, and (b) 0.84±0.07 (SeSMiK),0.54±0.13 (MRI), 0.59±0.19 (MRS), and 0.62±0.18 (COD) for distinguishing high and low grade CaP using a leave one out cross-validation strategy, all evaluations being performed on a per voxel basis. Our results suggest that following further rigorous validation, SeSMiK-GE could be developed into a powerful diagnostic and prognostic tool for detection and grading of CaP in vivo and in helping to determine the appropriate treatment option. Identifying low grade disease in vivo might allow CaP patients to opt for active surveillance rather than immediately opt for aggressive therapy such as radical prostatectomy.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures







References
-
- Ampeliotis D, Antonakoudi A, Berberidis K, Psarakis EZ, Kounoudes A. A computer-aided system for the detection of prostate cancer based on magnetic resonance image analysis. Communications, Control and Signal Processing. 2008:1372–1377.
-
- Bostwick DG. Grading prostate cancer. American Journal of Clinical Pathology. 1994;102:S38–S56. - PubMed
-
- Breiman L. Random forest. Machine Learning. 2001;451:5–32.
-
- Cai D, He X, Hu Y, Han J, Huang T. Learning a spatially smooth subspace for face recognition. Proc. IEEE Conf. Computer Vision and Pattern Recognition Machine Learning (CVPR’07) 2007:1–7.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

