Mouse vocal communication system: are ultrasounds learned or innate?
- PMID: 23295209
- PMCID: PMC3886250
- DOI: 10.1016/j.bandl.2012.10.002
Mouse vocal communication system: are ultrasounds learned or innate?
Abstract
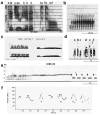
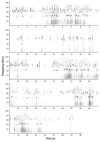
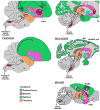
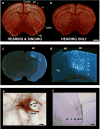
Mouse ultrasonic vocalizations (USVs) are often used as behavioral readouts of internal states, to measure effects of social and pharmacological manipulations, and for behavioral phenotyping of mouse models for neuropsychiatric and neurodegenerative disorders. However, little is known about the neurobiological mechanisms of rodent USV production. Here we discuss the available data to assess whether male mouse song behavior and the supporting brain circuits resemble those of known vocal non-learning or vocal learning species. Recent neurobiology studies have demonstrated that the mouse USV brain system includes motor cortex and striatal regions, and that the vocal motor cortex sends a direct sparse projection to the brainstem vocal motor nucleus ambiguous, a projection previously thought be unique to humans among mammals. Recent behavioral studies have reported opposing conclusions on mouse vocal plasticity, including vocal ontogeny changes in USVs over early development that might not be explained by innate maturation processes, evidence for and against a role for auditory feedback in developing and maintaining normal mouse USVs, and evidence for and against limited vocal imitation of song pitch. To reconcile these findings, we suggest that the trait of vocal learning may not be dichotomous but encompass a broad spectrum of behavioral and neural traits we call the continuum hypothesis, and that mice possess some of the traits associated with a capacity for limited vocal learning.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Aitken PG. Cortical control of conditioned and spontaneous vocal behavior in rhesus monkeys. Brain and Language. 1981;13(1):171–184. - PubMed
-
- Bass AH, McKibben JR. Neural mechanisms and behaviors for acoustic communication in teleost fish. Progress in Neurobiology. 2003;69(1):1–26. - PubMed
-
- Berquist SW, Ho JP, Metzner W. Sound production in the isolated mouse larynx. Society for Neuroscience Annual Meeting; San Diego. 2010. Aug 23,
-
- Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. The Journal of Comparative Neurology. 1989;279(2):312–326. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

