Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice
- PMID: 23295443
- PMCID: PMC3608818
- DOI: 10.1053/j.gastro.2012.12.028
Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice
Abstract
Background & aims: The endocannabinoid and eicosanoid lipid signaling pathways have important roles in inflammatory syndromes. Monoacylglycerol lipase (MAGL) links these pathways, hydrolyzing the endocannabinoid 2-arachidonoylglycerol to generate the arachidonic acid precursor pool for prostaglandin production. We investigated whether blocking MAGL protects against inflammation and damage from hepatic ischemia/reperfusion (I/R) and other insults.
Methods: We analyzed the effects of hepatic I/R in mice given the selective MAGL inhibitor JZL184, in Mgll(-/-) mice, fatty acid amide hydrolase(-/-) mice, and in cannabinoid receptor type 1(-/-) (CB1-/-) and cannabinoid receptor type 2(-/-) (CB2-/-). Liver tissues were collected and analyzed, along with cultured hepatocytes and Kupffer cells. We measured endocannabinoids, eicosanoids, and markers of inflammation, oxidative stress, and cell death using molecular biology, biochemistry, and mass spectrometry analyses.
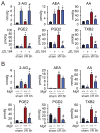
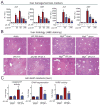
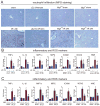
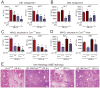
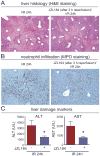
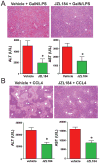
Results: Wild-type mice given JZL184 and Mgll(-/-) mice were protected from hepatic I/R injury by a mechanism that involved increased endocannabinoid signaling via CB2 and reduced production of eicosanoids in the liver. JZL184 suppressed the inflammation and oxidative stress that mediate hepatic I/R injury. Hepatocytes were the major source of hepatic MAGL activity and endocannabinoid and eicosanoid production. JZL184 also protected from induction of liver injury by D-(+)-galactosamine and lipopolysaccharides or CCl4.
Conclusions: MAGL modulates hepatic injury via endocannabinoid and eicosanoid signaling; blockade of this pathway protects mice from liver injury. MAGL inhibitors might be developed to treat conditions that expose the liver to oxidative stress and inflammatory damage.
Copyright © 2013 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Walsh KB, Toledo AH, Rivera-Chavez FA, et al. Inflammatory mediators of liver ischemia-reperfusion injury. Exp Clin Transplant. 2009;7:78–93. - PubMed
-
- Kobayashi M, Takeyoshi I, Kurabayashi M, et al. The effects of a cyclooxygenase-2 inhibitor, FK3311, on total hepatic ischemia-reperfusion injury of the rat. Hepatogastroenterology. 2007;54:522–526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

