Incretin hormones and the satiation signal
- PMID: 23295502
- PMCID: PMC3768099
- DOI: 10.1038/ijo.2012.208
Incretin hormones and the satiation signal
Abstract
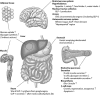
Recent research has indicated that appetite-regulating hormones from the gut may have therapeutic potential. The incretin hormone, glucagon-like peptide-1 (GLP-1), appears to be involved in both peripheral and central pathways mediating satiation. Several studies have also indicated that GLP-1 levels and responses to meals may be altered in obese subjects. Clinical trial results have shown further that two GLP-1 receptor agonists (GLP-1 RAs), exenatide and liraglutide, which are approved for the treatment of hyperglycemia in patients with type 2 diabetes, also produce weight loss in overweight subjects without diabetes. Thus, GLP-1 RAs may provide a new option for pharmacological treatment of obesity.
Figures


References
-
- Vilsboll T, Krarup T, Madsbad S, Holst JJ. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept. 2003;114:115–121. - PubMed
-
- Holst JJ, Deacon CF, Vilsboll T, Krarup T, Madsbad S. Glucagon-like peptide-1, glucose homeostasis and diabetes. Trends Mol Med. 2008;14:161–168. - PubMed
-
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. - PubMed
-
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. - PubMed
-
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

