Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma
- PMID: 23295794
- PMCID: PMC4878048
- DOI: 10.1200/JCO.2012.44.6112
Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma
Abstract
Purpose: In phase I/II trials, the cytotoxic T lymphocyte-associated antigen-4-blocking monoclonal antibody tremelimumab induced durable responses in a subset of patients with advanced melanoma. This phase III study evaluated overall survival (OS) and other safety and efficacy end points in patients with advanced melanoma treated with tremelimumab or standard-of-care chemotherapy.
Patients and methods: Patients with treatment-naive, unresectable stage IIIc or IV melanoma were randomly assigned at a ratio of one to one to tremelimumab (15 mg/kg once every 90 days) or physician's choice of standard-of-care chemotherapy (temozolomide or dacarbazine).
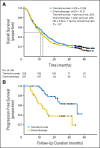
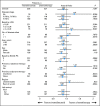
Results: In all, 655 patients were enrolled and randomly assigned. The test statistic crossed the prespecified futility boundary at second interim analysis after 340 deaths, but survival follow-up continued. At final analysis with 534 events, median OS by intent to treat was 12.6 months (95% CI, 10.8 to 14.3) for tremelimumab and 10.7 months (95% CI, 9.36 to 11.96) for chemotherapy (hazard ratio, 0.88; P = .127). Objective response rates were similar in the two arms: 10.7% in the tremelimumab arm and 9.8% in the chemotherapy arm. However, response duration (measured from date of random assignment) was significantly longer after tremelimumab (35.8 v 13.7 months; P = .0011). Diarrhea, pruritus, and rash were the most common treatment-related adverse events in the tremelimumab arm; 7.4% had endocrine toxicities. Seven deaths in the tremelimumab arm and one in the chemotherapy arm were considered treatment related by either investigators or sponsor.
Conclusion: This study failed to demonstrate a statistically significant survival advantage of treatment with tremelimumab over standard-of-care chemotherapy in first-line treatment of patients with metastatic melanoma.
Trial registration: ClinicalTrials.gov NCT00257205.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Is tremelimumab beneficial in advanced melanoma?J Clin Oncol. 2013 Aug 1;31(22):2835-6. doi: 10.1200/JCO.2013.49.6158. Epub 2013 Jun 10. J Clin Oncol. 2013. PMID: 23752103 No abstract available.
-
Reply to K.S. Wilson et al.J Clin Oncol. 2013 Aug 1;31(22):2836-7. doi: 10.1200/JCO.2013.50.2120. J Clin Oncol. 2013. PMID: 24058931 No abstract available.
References
-
- Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675206. J Clin Oncol. 2005;23:8968–8977. - PubMed
-
- Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27:1075–1081. - PubMed
-
- Ribas A, Hanson DC, Noe DA, et al. Tremelimumab (CP-675206), a cytotoxic T lymphocyte associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncologist. 2007;12:873–883. - PubMed
-
- Millham R, Pavlov D, Canniff P, et al. Ex vivo blood stimulation assay as a translational research tool in the development of the ticilimumab (CP-675206) J Clin Oncol. 2006;24(suppl):110s. abstr 2542.
-
- Kirkwood JM, Lorigan P, Hersey P, et al. Phase II trial of tremelimumab (CP-675206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res. 2010;16:1042–1048. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

