Patient income level and cancer clinical trial participation
- PMID: 23295802
- PMCID: PMC3565180
- DOI: 10.1200/JCO.2012.45.4553
Patient income level and cancer clinical trial participation
Abstract
Purpose: Studies have shown an association between socioeconomic status (SES) and quality of oncology care, but less is known about the impact of patient SES on clinical trial participation.
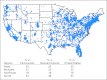
Patients and methods: We assessed clinical trial participation patterns according to important SES (income, education) and demographic factors in a large sample of patients surveyed via an Internet-based treatment decision tool. Logistic regression, conditioning on type of cancer, was used. Attitudes toward clinical trials were assessed using prespecified items about treatment, treatment tolerability, convenience, and cost.
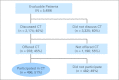
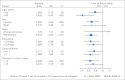
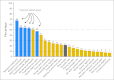
Results: From 2007 to 2011, 5,499 patients were successfully surveyed. Forty percent discussed clinical trials with their physician, 45% of discussions led to physician offers of clinical trial participation, and 51% of offers led to clinical trial participation. The overall clinical trial participation rate was 9%. In univariate models, older patients (P = .002) and patients with lower income (P = .001) and education (P = .02) were less likely to participate in clinical trials. In a multivariable model, income remained a statistically significant predictor of clinical trial participation (odds ratio, 0.73; 95% CI, 0.57 to 0.94; P = .01). Even in patients age ≥ 65 years, who have universal access to Medicare, lower income predicted lower trial participation. Cost concerns were much more evident among lower-income patients (P < .001).
Conclusion: Lower-income patients were less likely to participate in clinical trials, even when considering age group. A better understanding of why income is a barrier may help identify ways to make clinical trials better available to all patients and would increase the generalizability of clinical trial results across all income levels.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. - PubMed
-
- Tejeda HA, Green SB, Trimble EL, et al. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996;88:812–816. - PubMed
-
- Comis RL, Miller JD, Aldigé CR, et al. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21:830–835. - PubMed
-
- Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. - PubMed
-
- Hunter CP, Frelick RW, Feldman AR, et al. Selection factors in clinical trials: Results from the Community Clinical Oncology Program Physician's Patient Log. Cancer Treat Rep. 1987;71:559–565. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

