Reduced vancomycin susceptibility in an in vitro catheter-related biofilm model correlates with poor therapeutic outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus
- PMID: 23295925
- PMCID: PMC3591927
- DOI: 10.1128/AAC.02073-12
Reduced vancomycin susceptibility in an in vitro catheter-related biofilm model correlates with poor therapeutic outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus
Abstract
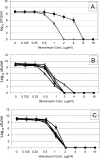
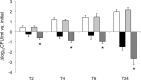
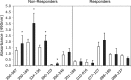
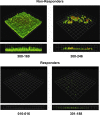
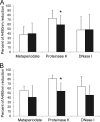
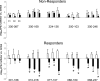
Staphylococcus aureus is the most common cause of endovascular infections, including catheter sepsis and infective endocarditis (IE). Vancomycin (VAN) is the primary choice for treatment of methicillin-resistant S. aureus (MRSA) infections. However, high rates of VAN treatment failure in MRSA infections caused by VAN-susceptible strains have been increasingly reported. Biofilm-associated MRSA infections are especially prone to clinical antibiotic failure. The present studies examined potential relationships between MRSA susceptibility to VAN in biofilms in vitro and nonsusceptibility to VAN in endovascular infection in vivo. Using 10 "VAN-susceptible" MRSA bloodstream isolates previously investigated for VAN responsiveness in experimental IE, we studied the mechanism(s) of such in vivo VAN resistance, including: (i) VAN binding to MRSA organisms; (ii) the impact of VAN on biofilm formation and biofilm composition; (iii) VAN efficacy in an in vitro catheter-related biofilm model; (iv) effects on cell wall thickness. As a group, the five strains previously categorized as VAN nonresponders (non-Rsp) in the experimental IE model differed from the five responders (Rsp) in terms of lower VAN binding, increased biofilm formation, higher survival in the presence of VAN within biofilms in the presence or absence of catheters, and greater biofilm reduction upon proteinase K treatment. Interestingly, sub-MICs of VAN significantly promoted biofilm formation only in the non-Rsp isolates. Cell wall thickness was similar among all MRSA strains. These results suggest that sublethal VAN levels that induce biofilm formation and reduce efficacy of VAN in the in vitro catheter-associated biofilms may contribute to suboptimal treatment outcomes for endovascular infections caused by "VAN-susceptible" MRSA strains.
Figures

References
-
- Kiedrowski MR, Horswill AR. 2011. New approaches for treating staphylococcal biofilm infections. Ann. N. Y. Acad. Sci. 1241:104–121 - PubMed
-
- Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, Rudrik JT, Pupp GR, Brown WJ, Cardo D, Fridkin SK. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342–1347 - PubMed
-
- Melchior MB, Fink-Gremmels J, Gaastra W. 2006. Comparative assessment of the antimicrobial susceptibility of Staphylococcus aureus isolates from bovine mastitis in biofilm versus planktonic culture. J. Vet. Med. 53:326–332 - PubMed
-
- Vlastarakos PV, Nikolopoulos TP, Maragoudakis P, Tzagaroulakis A, Ferekidis E. 2007. Biofilms in ear, nose, and throat infections: how important are they? Laryngoscope 117:668–673 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

