Activities of fosfomycin, tigecycline, colistin, and gentamicin against extended-spectrum-β-lactamase-producing Escherichia coli in a foreign-body infection model
- PMID: 23295934
- PMCID: PMC3591882
- DOI: 10.1128/AAC.01718-12
Activities of fosfomycin, tigecycline, colistin, and gentamicin against extended-spectrum-β-lactamase-producing Escherichia coli in a foreign-body infection model
Abstract
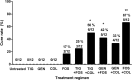
Limited antimicrobial agents are available for the treatment of implant-associated infections caused by fluoroquinolone-resistant Gram-negative bacilli. We compared the activities of fosfomycin, tigecycline, colistin, and gentamicin (alone and in combination) against a CTX-M15-producing strain of Escherichia coli (Bj HDE-1) in vitro and in a foreign-body infection model. The MIC and the minimal bactericidal concentration in logarithmic phase (MBC(log)) and stationary phase (MBC(stat)) were 0.12, 0.12, and 8 μg/ml for fosfomycin, 0.25, 32, and 32 μg/ml for tigecycline, 0.25, 0.5, and 2 μg/ml for colistin, and 2, 8, and 16 μg/ml for gentamicin, respectively. In time-kill studies, colistin showed concentration-dependent activity, but regrowth occurred after 24 h. Fosfomycin demonstrated rapid bactericidal activity at the MIC, and no regrowth occurred. Synergistic activity between fosfomycin and colistin in vitro was observed, with no detectable bacterial counts after 6 h. In animal studies, fosfomycin reduced planktonic counts by 4 log(10) CFU/ml, whereas in combination with colistin, tigecycline, or gentamicin, it reduced counts by >6 log(10) CFU/ml. Fosfomycin was the only single agent which was able to eradicate E. coli biofilms (cure rate, 17% of implanted, infected cages). In combination, colistin plus tigecycline (50%) and fosfomycin plus gentamicin (42%) cured significantly more infected cages than colistin plus gentamicin (33%) or fosfomycin plus tigecycline (25%) (P < 0.05). The combination of fosfomycin plus colistin showed the highest cure rate (67%), which was significantly better than that of fosfomycin alone (P < 0.05). In conclusion, the combination of fosfomycin plus colistin is a promising treatment option for implant-associated infections caused by fluoroquinolone-resistant Gram-negative bacilli.
Figures



References
-
- Sendi P, Zimmerli W. 2011. Challenges in periprosthetic knee-joint infection. Int. J. Artif. Organs 34:947–956 - PubMed
-
- Zimmerli W, Moser C. 2012. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol. Med. Microbiol. 65:158–168 - PubMed
-
- Moran E, Byren I, Atkins BL. 2010. The diagnosis and management of prosthetic joint infections. J. Antimicrob. Chemother. 65(Suppl. 3):iii45–iii54 - PubMed
-
- Trampuz A, Widmer AF. 2006. Infections associated with orthopedic implants. Curr. Opin. Infect. Dis. 19:349–356 - PubMed
-
- Martinez-Pastor JC, Munoz-Mahamud E, Vilchez F, Garcia-Ramiro S, Bori G, Sierra J, Martinez JA, Font L, Mensa J, Soriano A. 2009. Outcome of acute prosthetic joint infections due to gram-negative bacilli treated with open debridement and retention of the prosthesis. Antimicrob. Agents Chemother. 53:4772–4777 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

