Intramuscular scAAV9-SMN injection mediates widespread gene delivery to the spinal cord and decreases disease severity in SMA mice
- PMID: 23295949
- PMCID: PMC3594018
- DOI: 10.1038/mt.2012.261
Intramuscular scAAV9-SMN injection mediates widespread gene delivery to the spinal cord and decreases disease severity in SMA mice
Abstract
We have recently demonstrated the remarkable efficiency of self-complementary (sc) AAV9 vectors for central nervous system (CNS) gene transfer following intravenous delivery in mice and larger animals. Here, we investigated whether gene delivery to motor neurons (MNs) could also be achieved via intramuscular (i.m.) scAAV9 injection and subsequent retrograde transport along the MNs axons. Unexpectedly, we found that a single injection of scAAV9 into the adult mouse gastrocnemius (GA) mediated widespread MN transduction along the whole spinal cord, without limitation to the MNs connected to the injected muscle. Spinal cord astrocytes and peripheral organs were also transduced, indicating vector spread from the injected muscle to both the CNS and the periphery through release into the blood circulation. Moreover, we showed that i.m. injection of scAAV9 vectors expressing "survival of motor neuron" (Smn) in spinal muscular atrophy (SMA) mice mediated high survival motor neuron (SMN) expression levels at both the CNS and the periphery, and increased the median lifespan from 12 days to 163 days. These findings represent to date the longest extent in survival obtained in SMA mice following i.m. viral vector gene delivery, and might generate a renewed interest in the use of i.m. adeno-associated viruses (AAV) delivery for the development of gene therapy strategies for MN diseases.
Figures
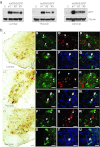
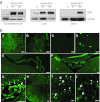





References
-
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM.et al. (2004VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model Nature 429413–417. - PubMed
-
- Baumgartner BJ., and, Shine HD. Neuroprotection of spinal motoneurons following targeted transduction with an adenoviral vector carrying the gene for glial cell line-derived neurotrophic factor. Exp Neurol. 1998;153:102–112. - PubMed
-
- Gimenez y Ribotta M, Revah F, Pradier L, Loquet I, Mallet J, Privat A. Prevention of motoneuron death by adenovirus-mediated neurotrophic factors. J Neurosci Res. 1997;48:281–285. - PubMed
-
- Kaspar BK, Lladó J, Sherkat N, Rothstein JD., and, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

