A new model for allosteric regulation of phenylalanine hydroxylase: implications for disease and therapeutics
- PMID: 23296088
- PMCID: PMC3580015
- DOI: 10.1016/j.abb.2012.12.017
A new model for allosteric regulation of phenylalanine hydroxylase: implications for disease and therapeutics
Abstract
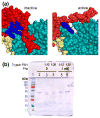
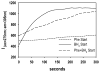
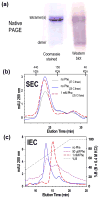
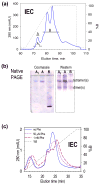
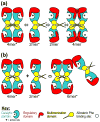
The structural basis for allosteric regulation of phenylalanine hydroxylase (PAH), whose dysfunction causes phenylketonuria (PKU), is poorly understood. A new morpheein model for PAH allostery is proposed to consist of a dissociative equilibrium between two architecturally different tetramers whose interconversion requires a ∼90° rotation between the PAH catalytic and regulatory domains, the latter of which contains an ACT domain. This unprecedented model is supported by in vitro data on purified full length rat and human PAH. The conformational change is both predicted to and shown to render the tetramers chromatographically separable using ion exchange methods. One novel aspect of the activated tetramer model is an allosteric phenylalanine binding site at the intersubunit interface of ACT domains. Amino acid ligand-stabilized ACT domain dimerization follows the multimerization and ligand binding behavior of ACT domains present in other proteins in the PDB. Spectroscopic, chromatographic, and electrophoretic methods demonstrate a PAH equilibrium consisting of two architecturally distinct tetramers as well as dimers. We postulate that PKU-associated mutations may shift the PAH quaternary structure equilibrium in favor of the low activity assemblies. Pharmacological chaperones that stabilize the ACT:ACT interface can potentially provide PKU patients with a novel small molecule therapeutic.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




References
-
- Blau N, van Spronsen FJ, Levy HL. Lancet. 2010;376:1417–1427. - PubMed
-
- Antshel KM. Mol Genet Metab. 2010;99(Suppl 1):S52–58. - PubMed
-
- Gentile JK, Ten Hoedt AE, Bosch AM. Mol Genet Metab. 2010;99(Suppl 1):S64–67. - PubMed
-
- van Spronsen FJ, Enns GM. Mol Genet Metab. 2010;99(Suppl 1):S90–95. - PubMed
-
- Torreblanca R, Lira-Navarrete E, Sancho J, Hurtado-Guerrero R. Chembiochem. 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

