Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy
- PMID: 23296339
- PMCID: PMC3726719
- DOI: 10.1038/nrneurol.2012.263
Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy
Erratum in
- Nat Rev Neurol. 2013. doi: 10.1038/nmeurol.2013.32. Liu, Chia-Chan [corrected to Liu, Chia-Chen] doi: 10.1038/nmeurol.2013.32
Abstract
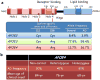
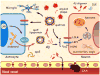
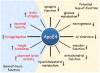
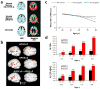
Apolipoprotein E (Apo-E) is a major cholesterol carrier that supports lipid transport and injury repair in the brain. APOE polymorphic alleles are the main genetic determinants of Alzheimer disease (AD) risk: individuals carrying the ε4 allele are at increased risk of AD compared with those carrying the more common ε3 allele, whereas the ε2 allele decreases risk. Presence of the APOE ε4 allele is also associated with increased risk of cerebral amyloid angiopathy and age-related cognitive decline during normal ageing. Apo-E-lipoproteins bind to several cell-surface receptors to deliver lipids, and also to hydrophobic amyloid-β (Aβ) peptide, which is thought to initiate toxic events that lead to synaptic dysfunction and neurodegeneration in AD. Apo-E isoforms differentially regulate Aβ aggregation and clearance in the brain, and have distinct functions in regulating brain lipid transport, glucose metabolism, neuronal signalling, neuroinflammation, and mitochondrial function. In this Review, we describe current knowledge on Apo-E in the CNS, with a particular emphasis on the clinical and pathological features associated with carriers of different Apo-E isoforms. We also discuss Aβ-dependent and Aβ-independent mechanisms that link Apo-E4 status with AD risk, and consider how to design effective strategies for AD therapy by targeting Apo-E.
Figures
References
-
- Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimer’s dement. 2012;8:131–168. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. - PubMed
-
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. - PubMed
-
- Roberson ED, et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

