Sickle hemoglobin disturbs normal coupling among erythrocyte O2 content, glycolysis, and antioxidant capacity
- PMID: 23297128
- PMCID: PMC3587328
- DOI: 10.1182/blood-2012-02-414037
Sickle hemoglobin disturbs normal coupling among erythrocyte O2 content, glycolysis, and antioxidant capacity
Abstract
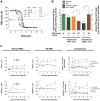
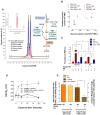
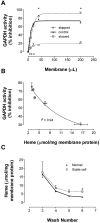
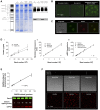
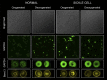
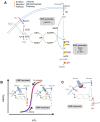
Energy metabolism in RBCs is characterized by O2-responsive variations in flux through the Embden Meyerhof pathway (EMP) or the hexose monophosphate pathway (HMP). Therefore, the generation of ATP, NADH, and 2,3-DPG (EMP) or NADPH (HMP) shift with RBC O2 content because of competition between deoxyhemoglobin and key EMP enzymes for binding to the cytoplasmic domain of the Band 3 membrane protein (cdB3). Enzyme inactivation by cdB3 sequestration in oxygenated RBCs favors HMP flux and NADPH generation (maximizing glutathione-based antioxidant systems). We tested the hypothesis that sickle hemoglobin disrupts cdB3-based regulatory protein complex assembly, creating vulnerability to oxidative stress. In RBCs from patients with sickle cell anemia, we demonstrate in the present study constrained HMP flux, NADPH, and glutathione recycling and reduced resilience to oxidative stress manifested by membrane protein oxidation and membrane fragility. Using a novel, inverted membrane-on-bead model, we illustrate abnormal (O2-dependent) association of sickle hemoglobin to RBC membrane that interferes with sequestration/inactivation of the EMP enzyme GAPDH. This finding was confirmed by immunofluorescent imaging during RBC O2 loading/unloading. Moreover, selective inhibition of inappropriately dispersed GAPDH rescues antioxidant capacity. Such disturbance of cdB3-based linkage between O2 gradients and RBC metabolism suggests a novel mechanism by which hypoxia may influence the sickle cell anemia phenotype.
Figures

References
-
- Embury SH. The not-so-simple process of sickle cell vasoocclusion. Microcirculation. 2004;11(2):101–113. - PubMed
-
- Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life. 2012;64(1):72–80. - PubMed
-
- Riggs A, Wells M. The oxygen equilibrium of sickle-cell hemoglobin. Biochim Biophys Acta. 1961;50:243–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

