Calcium oscillations-coupled conversion of actin travelling waves to standing oscillations
- PMID: 23297209
- PMCID: PMC3557052
- DOI: 10.1073/pnas.1221538110
Calcium oscillations-coupled conversion of actin travelling waves to standing oscillations
Abstract
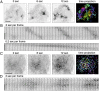
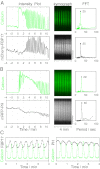
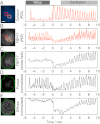
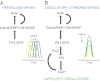
Dynamic spatial patterns of signaling factors or macromolecular assemblies in the form of oscillations or traveling waves have emerged as important themes in cell physiology. Feedback mechanisms underlying these processes and their modulation by signaling events and reciprocal cross-talks remain poorly understood. Here we show that antigen stimulation of mast cells triggers cyclic changes in the concentration of actin regulatory proteins and actin in the cell cortex that can be manifested in either spatial pattern. Recruitment of FBP17 and active Cdc42 at the plasma membrane, leading to actin polymerization, are involved in both events, whereas calcium oscillations, which correlate with global fluctuations of plasma membrane PI(4,5)P(2), are tightly linked to standing oscillations and counteract wave propagation. These findings demonstrate the occurrence of a calcium-independent oscillator that controls the collective dynamics of factors linking the actin cytoskeleton to the plasma membrane. Coupling between this oscillator and the one underlying global plasma membrane PI(4,5)P2 and calcium oscillations spatially regulates actin dynamics, revealing an unexpected pattern-rendering mechanism underlying plastic changes occurring in the cortical region of the cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ozbudak EM, Becskei A, van Oudenaarden A. A system of counteracting feedback loops regulates Cdc42p activity during spontaneous cell polarization. Dev Cell. 2005;9(4):565–571. - PubMed
-
- Giannone G, et al. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116(3):431–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

