Spatial regulation of UBXD8 and p97/VCP controls ATGL-mediated lipid droplet turnover
- PMID: 23297223
- PMCID: PMC3557085
- DOI: 10.1073/pnas.1213738110
Spatial regulation of UBXD8 and p97/VCP controls ATGL-mediated lipid droplet turnover
Abstract
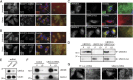
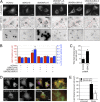
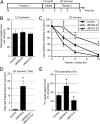
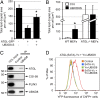
UBXD8 is a membrane-embedded recruitment factor for the p97/VCP segregase that has been previously linked to endoplasmic reticulum (ER)-associated degradation and to the control of triacylglycerol synthesis in the ER. UBXD8 also has been identified as a component of cytoplasmic lipid droplets (LDs), but neither the mechanisms that control its trafficking between the ER and LDs nor its functions in the latter organelle have been investigated previously. Here we report that association of UBXD8 with the ER-resident rhomboid pseudoprotease UBAC2 specifically restricts trafficking of UBXD8 to LDs, and that the steady-state partitioning of UBXD8 between the ER and LDs can be experimentally manipulated by controlling the relative expression of these two proteins. We exploit this interaction to show that UBXD8-mediated recruitment of p97/VCP to LDs increases LD size by inhibiting the activity of adipose triglyceride lipase (ATGL), the rate-limiting enzyme in triacylglycerol hydrolysis. Our findings show that UBXD8 binds directly to ATGL and promotes dissociation of its endogenous coactivator, CGI-58. These data indicate that UBXD8 and p97/VCP play central integrative roles in cellular energy homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

