Cyanobacterial conversion of carbon dioxide to 2,3-butanediol
- PMID: 23297225
- PMCID: PMC3557092
- DOI: 10.1073/pnas.1213024110
Cyanobacterial conversion of carbon dioxide to 2,3-butanediol
Abstract
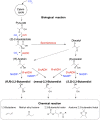
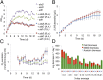
Conversion of CO(2) for the synthesis of chemicals by photosynthetic organisms is an attractive target for establishing independence from fossil reserves. However, synthetic pathway construction in cyanobacteria is still in its infancy compared with model fermentative organisms. Here we systematically developed the 2,3-butanediol (23BD) biosynthetic pathway in Synechococcus elongatus PCC7942 as a model system to establish design methods for efficient exogenous chemical production in cyanobacteria. We identified 23BD as a target chemical with low host toxicity, and designed an oxygen-insensitive, cofactor-matched biosynthetic pathway coupled with irreversible enzymatic steps to create a driving force toward the target. Production of 23BD from CO(2) reached 2.38 g/L, which is a significant increase for chemical production from exogenous pathways in cyanobacteria. This work demonstrates that developing strong design methods can continue to increase chemical production in cyanobacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- McFarlane J, Robinson S. Survey of Alternative Feedstocks for Commodity Chemical Manufacturing. TN: Oak Ridge National Laboratory; 2007.
-
- U.S. Energy Information Administration (2009) Annual Energy Review 2008. Available at http://www.eia.gov/totalenergy/data/annual/previous.cfm. Accessed December 21, 2012.
-
- Herzog H, Golomb D. In: Encyclopedia of Energy. Cutler JC, editor. New York: Elsevier; 2004. pp. 277–287.
-
- Ruffing AM. Engineered cyanobacteria: Teaching an old bug new tricks. Bioeng Bugs. 2011;2(3):136–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

