Cryptosporidium pathogenicity and virulence
- PMID: 23297262
- PMCID: PMC3553671
- DOI: 10.1128/CMR.00076-12
Cryptosporidium pathogenicity and virulence
Abstract
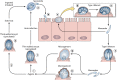
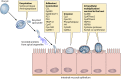
Cryptosporidium is a protozoan parasite of medical and veterinary importance that causes gastroenteritis in a variety of vertebrate hosts. Several studies have reported different degrees of pathogenicity and virulence among Cryptosporidium species and isolates of the same species as well as evidence of variation in host susceptibility to infection. The identification and validation of Cryptosporidium virulence factors have been hindered by the renowned difficulties pertaining to the in vitro culture and genetic manipulation of this parasite. Nevertheless, substantial progress has been made in identifying putative virulence factors for Cryptosporidium. This progress has been accelerated since the publication of the Cryptosporidium parvum and C. hominis genomes, with the characterization of over 25 putative virulence factors identified by using a variety of immunological and molecular techniques and which are proposed to be involved in aspects of host-pathogen interactions from adhesion and locomotion to invasion and proliferation. Progress has also been made in the contribution of host factors that are associated with variations in both the severity and risk of infection. Here we provide a review comprised of the current state of knowledge on Cryptosporidium infectivity, pathogenesis, and transmissibility in light of our contemporary understanding of microbial virulence.
Figures






References
-
- Alonso-Monge R, Navarro-García F, Román E, Eisman B, Nombela C, Pla J. 2003. Strategies for the identification of virulence determinants in human pathogenic fungi. Curr. Genet. 42:301–312 - PubMed
-
- Ebert D, Herre EA. 1996. The evolution of parasitic diseases. Parasitol. Today 12:96–101 - PubMed
-
- Garnick E. 1992. Parasite virulence and parasite-host coevolution: a reappraisal. J. Parasitol. 78:381–386 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

