The Ebola virus matrix protein penetrates into the plasma membrane: a key step in viral protein 40 (VP40) oligomerization and viral egress
- PMID: 23297401
- PMCID: PMC3581432
- DOI: 10.1074/jbc.M112.443960
The Ebola virus matrix protein penetrates into the plasma membrane: a key step in viral protein 40 (VP40) oligomerization and viral egress
Abstract
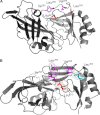
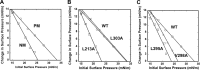
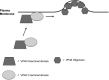
Ebola, a fatal virus in humans and non-human primates, has no Food and Drug Administration-approved vaccines or therapeutics. The virus from the Filoviridae family causes hemorrhagic fever, which rapidly progresses and in some cases has a fatality rate near 90%. The Ebola genome encodes seven genes, the most abundantly expressed of which is viral protein 40 (VP40), the major Ebola matrix protein that regulates assembly and egress of the virus. It is well established that VP40 assembles on the inner leaflet of the plasma membrane; however, the mechanistic details of plasma membrane association by VP40 are not well understood. In this study, we used an array of biophysical experiments and cellular assays along with mutagenesis of VP40 to investigate the role of membrane penetration in VP40 assembly and egress. Here we demonstrate that VP40 is able to penetrate specifically into the plasma membrane through an interface enriched in hydrophobic residues in its C-terminal domain. Mutagenesis of this hydrophobic region consisting of Leu(213), Ile(293), Leu(295), and Val(298) demonstrated that membrane penetration is critical to plasma membrane localization, VP40 oligomerization, and viral particle egress. Taken together, VP40 membrane penetration is an important step in the plasma membrane localization of the matrix protein where oligomerization and budding are defective in the absence of key hydrophobic interactions with the membrane.
Figures






References
-
- Johnson K. M., Lange J. V., Webb P. A., Murphy F. A. (1977) Isolation and partial characterisation of a new virus causing acute haemorrhagic fever in Zaire. Lancet 1, 569–571 - PubMed
-
- Elliott L. H., Kiley M. P., McCormick J. B. (1985) Descriptive analysis of Ebola virus proteins. Virology 147, 169–176 - PubMed
-
- Feldmann H., Volchkov V. E., Volchkova V. A., Klenk H. D. (1999) The glycoproteins of Marburg and Ebola virus and their potential roles in pathogenesis. Arch. Virol. Suppl. 15, 159–169 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

