Brain damage in methylmalonic aciduria: 2-methylcitrate induces cerebral ammonium accumulation and apoptosis in 3D organotypic brain cell cultures
- PMID: 23298464
- PMCID: PMC3567978
- DOI: 10.1186/1750-1172-8-4
Brain damage in methylmalonic aciduria: 2-methylcitrate induces cerebral ammonium accumulation and apoptosis in 3D organotypic brain cell cultures
Abstract
Background: Methylmalonic aciduria is an inborn error of metabolism characterized by accumulation of methylmalonate (MMA), propionate and 2-methylcitrate (2-MCA) in body fluids. Early diagnosis and current treatment strategies aimed at limiting the production of these metabolites are only partially effective in preventing neurological damage.
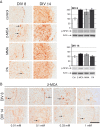
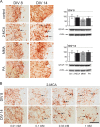
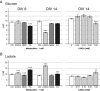
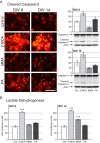
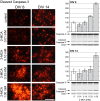
Methods: To explore the metabolic consequences of methylmalonic aciduria on the brain, we used 3D organotypic brain cell cultures from rat embryos. We challenged the cultures at two different developmental stages with 1 mM MMA, propionate or 2-MCA applied 6 times every 12 h. In a dose-response experiment cultures were challenged with 0.01, 0.1, 0.33 and 1 mM 2-MCA. Immunohistochemical staining for different brain cell markers were used to assess cell viability, morphology and differentiation. Significant changes were validated by western blot analysis. Biochemical markers were analyzed in culture media. Apoptosis was studied by immunofluorescence staining and western blots for activated caspase-3.
Results: Among the three metabolites tested, 2-MCA consistently produced the most pronounced effects. Exposure to 2-MCA caused morphological changes in neuronal and glial cells already at 0.01 mM. At the biochemical level the most striking result was a significant ammonium increase in culture media with a concomitant glutamine decrease. Dose-response studies showed significant and parallel changes of ammonium and glutamine starting from 0.1 mM 2-MCA. An increased apoptosis rate was observed by activation of caspase-3 after exposure to at least 0.1 mM 2-MCA.
Conclusion: Surprisingly, 2-MCA, and not MMA, seems to be the most toxic metabolite in our in vitro model leading to delayed axonal growth, apoptosis of glial cells and to unexpected ammonium increase. Morphological changes were already observed at 2-MCA concentrations as low as 0.01 mM. Increased apoptosis and ammonium accumulation started at 0.1 mM thus suggesting that ammonium accumulation is secondary to cell suffering and/or cell death. Local accumulation of ammonium in CNS, that may remain undetected in plasma and urine, may therefore play a key role in the neuropathogenesis of methylmalonic aciduria both during acute decompensations and in chronic phases. If confirmed in vivo, this finding might shift the current paradigm and result in novel therapeutic strategies.
Figures



References
-
- Lempp TJ, Suormala T, Siegenthaler R, Baumgartner ER, Fowler B, Steinmann B, Baumgartner MR. Mutation and biochemical analysis of 19 probands with mut0 and 13 with mut- methylmalonic aciduria: identification of seven novel mutations. Mol Genet Metab. 2007;90:284–290. doi: 10.1016/j.ymgme.2006.10.002. - DOI - PubMed
-
- Dobson CM, Wai T, Leclerc D, Wilson A, Wu X, Dore C, Hudson T, Rosenblatt DS, Gravel RA. Identification of the gene responsible for the cblA complementation group of vitamin B12-responsive methylmalonic acidemia based on analysis of prokaryotic gene arrangements. Proc Natl Acad Sci U S A. 2002;99:15554–15559. doi: 10.1073/pnas.242614799. - DOI - PMC - PubMed
-
- Dobson CM, Wai T, Leclerc D, Kadir H, Narang M, Lerner-Ellis JP, Hudson TJ, Rosenblatt DS, Gravel RA. Identification of the gene responsible for the cblB complementation group of vitamin B12-dependent methylmalonic aciduria. Hum Mol Genet. 2002;11:3361–3369. doi: 10.1093/hmg/11.26.3361. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

