Co-enrollment of critically ill patients into multiple studies: patterns, predictors and consequences
- PMID: 23298553
- PMCID: PMC4056073
- DOI: 10.1186/cc11917
Co-enrollment of critically ill patients into multiple studies: patterns, predictors and consequences
Abstract
Introduction: Research on co-enrollment practices and their impact are limited in the ICU setting. The objectives of this study were: 1) to describe patterns and predictors of co-enrollment of patients in a thromboprophylaxis trial, and 2) to examine the consequences of co-enrollment on clinical and trial outcomes.
Methods: In an observational analysis of an international thromboprophylaxis trial in 67 ICUs, we examined the co-enrollment of critically ill medical-surgical patients into more than one study, and examined the clinical and trial outcomes among co-enrolled and non-co-enrolled patients.
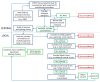
Results: Among 3,746 patients enrolled in PROTECT (Prophylaxis for ThromboEmbolism in Critical Care Trial), 713 (19.0%) were co-enrolled in at least one other study (53.6% in a randomized trial, 37.0% in an observational study and 9.4% in both). Six factors independently associated with co-enrollment (all P < 0.001) were illness severity (odds ratio (OR) 1.35, 95% confidence interval (CI) 1.19 to 1.53 for each 10-point Acute Physiology and Chronic Health Evaluation (APACHE) II score increase), substitute decision-makers providing consent, rather than patients (OR 3.31, 2.03 to 5.41), experience of persons inviting consent (OR 2.67, 1.74 to 4.11 for persons with > 10 years' experience compared to persons with none), center size (all ORs > 10 for ICUs with > 15 beds), affiliation with trials groups (OR 5.59, 3.49 to 8.95), and main trial rather than pilot phase (all ORs > 8 for recruitment year beyond the pilot). Co-enrollment did not influence clinical or trial outcomes or risk of adverse events.
Conclusions: Co-enrollment was strongly associated with features of the patients, research personnel, setting and study. Co-enrollment had no impact on trial results, and appeared safe, acceptable and feasible. Transparent reporting, scholarly discourse, ethical analysis and further research are needed on the complex topic of co-enrollment during critical illness.
Figures
References
-
- Abdool Karim Q, Kharsany AB, Naidoo K, Yende N, Gengiah T, Omar Z, Arulappan N, Mlisana KP, Luthuli LR, Abdool Karim SS. Co-enrollment in multiple HIV prevention trials - experiences from the CAPRISA 004 Tenofovir gel trial. Contemp Clin Trials. 2011;17:333–338. doi: 10.1016/j.cct.2011.01.005. - DOI - PMC - PubMed
-
- Cook DJ, Blythe D, Rischbieth A, Hebert PC, Zytaruk N, Menon K, Erikson S, Fowler R, Heels-Ansdell D, Meade MO. The Canadian Critical Care Trials Group. Enrolment of ICU patients into clinical studies: a tri-national survey. Crit Care Med. 2008;17:51–56. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

