Health care costs and resource utilization, including patient burden, associated with novel-agent-based treatment versus other therapies for multiple myeloma: findings using real-world claims data
- PMID: 23299776
- PMCID: PMC3556254
- DOI: 10.1634/theoncologist.2012-0113
Health care costs and resource utilization, including patient burden, associated with novel-agent-based treatment versus other therapies for multiple myeloma: findings using real-world claims data
Abstract
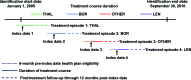
Background: . Treatment of multiple myeloma has dramatically improved with the introduction of bortezomib (BOR), thalidomide (THAL), and lenalidomide (LEN). Studies assessing health care costs, particularly economic burden on patients, are limited. We conducted a claims-based, retrospective analysis of total health care costs as well as patient burden (patient out-of-pocket costs and number of ambulatory/hospital visits) associated with BOR/THAL/LEN treatment versus other therapies (OTHER). METHODS. Treatment episodes starting between January 1, 2005 and September 30, 2010 were identified from the claims database of a large U.S. health plan. Health care costs and utilization were measured during 1 year after initiation and analyzed per treatment episode. Multivariate analyses were used to adjust for patient characteristics, comorbidities, and line of treatment.
Results: A total of 4,836 treatment episodes were identified. Mean adjusted total costs were similar between BOR ($112,889) and OTHER ($111,820), but higher with THAL ($129,412) and LEN ($158,428). Mean adjusted patient out-of-pocket costs were also similar for BOR ($3,846) and OTHER ($3,900) but remained higher with THAL ($4,666) and LEN ($4,483). Mean adjusted rates of ambulatory visits were similar across therapies (BOR: 69.67; THAL: 66.31; LEN: 65.60;
Other: 69.42).
Conclusions: Adjusted analyses of real-world claims data show that total health care costs, as well as patient out-of-pocket costs, are higher with THAL/LEN treatment episodes than with BOR/OTHER therapies. Additionally, similar rates of ambulatory visits suggest that any perceived advantage in patient convenience of the orally administered drugs THAL/LEN is not supported by these data.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
Comment in
-
Efficient allocation of novel agents in multiple myeloma: a work in progress.Oncologist. 2013;18(1):5-7. doi: 10.1634/theoncologist.2012-0484. Epub 2013 Jan 8. Oncologist. 2013. PMID: 23299778 Free PMC article.
References
-
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008. [Accessed October 20, 2012]. Available at http://seer.cancer.gov/csr/1975_2008/
-
- Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. - PubMed
-
- Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

