A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy
- PMID: 23299940
- PMCID: PMC3590982
- DOI: 10.1038/emboj.2012.340
A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy
Abstract
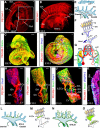
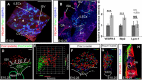
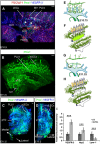
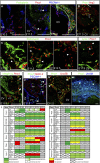
During mammalian development, a subpopulation of endothelial cells in the cardinal vein (CV) expresses lymphatic-specific genes and subsequently develops into the first lymphatic structures, collectively termed as lymph sacs. Budding, sprouting and ballooning of lymphatic endothelial cells (LECs) have been proposed to underlie the emergence of LECs from the CV, but the exact mechanisms of lymph vessel formation remain poorly understood. Applying selective plane illumination-based ultramicroscopy to entire wholemount-immunostained mouse embryos, we visualized the complete developing vascular system with cellular resolution. Here, we report emergence of the earliest detectable LECs as strings of loosely connected cells between the CV and superficial venous plexus. Subsequent aggregation of LECs resulted in formation of two distinct, previously unidentified lymphatic structures, the dorsal peripheral longitudinal lymphatic vessel (PLLV) and the ventral primordial thoracic duct (pTD), which at later stages formed a direct contact with the CV. Providing new insights into their function, we found vascular endothelial growth factor C (VEGF-C) and the matrix component CCBE1 indispensable for LEC budding and migration. Altogether, we present a significantly more detailed view and novel model of early lymphatic development.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Axnick J, Lammert E (2012) Vascular lumen formation. Curr Opin Hematol 19: 192–198 - PubMed
-
- Becker K, Jahrling N, Kramer ER, Schnorrer F, Dodt HU (2008) Ultramicroscopy: 3D reconstruction of large microscopical specimens. J Biophotonics 1: 36–42 - PubMed
-
- Blum Y, Belting HG, Ellertsdottir E, Herwig L, Luders F, Affolter M (2008) Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol 316: 312–322 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

