Finding the end: recruitment of telomerase to telomeres
- PMID: 23299958
- PMCID: PMC3805138
- DOI: 10.1038/nrm3505
Finding the end: recruitment of telomerase to telomeres
Abstract
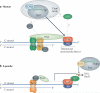
Telomeres, the ends of linear eukaryotic chromosomes, are characterized by the presence of multiple repeats of a short DNA sequence. This telomeric DNA is protected from illicit repair by telomere-associated proteins, which in mammals form the shelterin complex. Replicative polymerases are unable to synthesize DNA at the extreme ends of chromosomes, but in unicellular eukaryotes such as yeast and in mammalian germ cells and stem cells, telomere length is maintained by a ribonucleoprotein enzyme known as telomerase. Recent work has provided insights into the mechanisms of telomerase recruitment to telomeres, highlighting the contribution of telomere-associated proteins, including TPP1 in humans, Ccq1 in Schizosaccharomyces pombe and Cdc13 and Ku70-Ku80 in Saccharomyces cerevisiae.
Figures



References
-
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. - PubMed
-
- Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225:951–960. - PubMed
-
- Pardue ML, DeBaryshe PG. Drosophila telomeres: A variation on the telomerase theme. Fly (Austin) 2008;2:101–110. - PubMed
-
- Dokal I. Dyskeratosis congenita. Hematology Am Soc Hematol Educ Program. 2011;2011:480–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

