An in vitro model of human acute ethanol exposure that incorporates CXCR3- and CXCR4-dependent recruitment of immune cells
- PMID: 23300006
- PMCID: PMC3576009
- DOI: 10.1093/toxsci/kfs337
An in vitro model of human acute ethanol exposure that incorporates CXCR3- and CXCR4-dependent recruitment of immune cells
Abstract
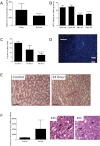
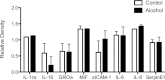
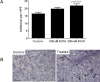
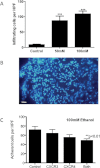
Alcoholic liver disease (ALD) is one of the commonest causes of cirrhosis and liver failure in the developed world. Hepatic inflammation is the critical stage in progression of both ALD and non-ALD, but it remains difficult to study the underlying mechanisms in a human system, and current animal models do not fully recapitulate human liver disease. We developed a human tissue-based system to study lymphocyte recruitment in response to ethanol challenge. Precision-cut liver slices (PCLS) from human livers were incubated in culture, and hepatic function was determined by albumin production, 3-(4,5-dimethylthiazol)-2,5-diphenyl tetrazolium bromide assay, glucose uptake responses, and morphometric assessment. Responses of tissue and lymphocytes to ethanol exposure were determined by PCR, flow cytometry, histology, and lymphocyte infiltration assays. Human PCLS demonstrated appropriate upregulation of CYP2E1, ADH1α, and ADH3 in response to ethanol treatment. Ethanol also induced expression of endothelial VCAM-1 and ICAM-1, production of sICAM-1 and CXCL8, and the chemokine receptors CXCR3 and CXCR4 on CD4 and CD8 lymphocytes. CXCR3- and CXCR4-dependent migration of lymphocytes into the tissue increased significantly in response to treatment with ethanol. We have demonstrated that ethanol increases chemokine receptor expression and lymphocyte recruitment into human liver tissue, suggesting that it may operate directly to promote hepatitis in ALD. The physiological and pathophysiological responses of the PCLS to ethanol in vitro highlight the potential of this assay for dissecting the molecular mechanisms underlying human liver inflammation and as a screening tool for novel therapeutics.
Figures


Similar articles
-
Liver tissue metabolically transformed by alcohol induces immune recognition of liver self-proteins but not in vivo inflammation.Am J Physiol Gastrointest Liver Physiol. 2018 Mar 1;314(3):G418-G430. doi: 10.1152/ajpgi.00183.2017. Epub 2017 Dec 28. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29351393 Free PMC article.
-
Differential expression of chemokines and chemokine receptors shapes the inflammatory response in rejecting human liver transplants.Transplantation. 2001 Dec 27;72(12):1957-67. doi: 10.1097/00007890-200112270-00016. Transplantation. 2001. PMID: 11773896
-
Adhesion of human haematopoietic (CD34+) stem cells to human liver compartments is integrin and CD44 dependent and modulated by CXCR3 and CXCR4.J Hepatol. 2009 Oct;51(4):734-49. doi: 10.1016/j.jhep.2009.06.021. Epub 2009 Jul 30. J Hepatol. 2009. PMID: 19703720
-
The chemokine receptors CXCR4 and CXCR3 in cancer.Curr Oncol Rep. 2009 Mar;11(2):125-31. doi: 10.1007/s11912-009-0019-1. Curr Oncol Rep. 2009. PMID: 19216844 Review.
-
Review: Precision Cut Liver Slices for the Evaluation of Fatty Liver and Fibrosis.Curr Mol Pharmacol. 2017;10(3):249-254. doi: 10.2174/1874467208666150817112345. Curr Mol Pharmacol. 2017. PMID: 26278387 Review.
Cited by
-
Emerging Importance of Chemokine Receptor CXCR4 and Its Ligand in Liver Disease.Front Cell Dev Biol. 2021 Jul 27;9:716842. doi: 10.3389/fcell.2021.716842. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34386499 Free PMC article. Review.
-
Profile analysis of hepatic porcine and murine brain tissue slices obtained with a vibratome.PeerJ. 2015 Apr 30;3:e932. doi: 10.7717/peerj.932. eCollection 2015. PeerJ. 2015. PMID: 25945319 Free PMC article.
-
Dysregulated hepatic expression of glucose transporters in chronic disease: contribution of semicarbazide-sensitive amine oxidase to hepatic glucose uptake.Am J Physiol Gastrointest Liver Physiol. 2014 Dec 15;307(12):G1180-90. doi: 10.1152/ajpgi.00377.2013. Epub 2014 Oct 23. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 25342050 Free PMC article.
-
Precision-cut human liver slice cultures as an immunological platform.J Immunol Methods. 2018 Apr;455:71-79. doi: 10.1016/j.jim.2018.01.012. Epub 2018 Feb 1. J Immunol Methods. 2018. PMID: 29408707 Free PMC article.
-
Involvement of Cxcl12a/Cxcr4b Chemokine System in Mediating the Stimulatory Effect of Embryonic Ethanol Exposure on Neuronal Density in Zebrafish Hypothalamus.Alcohol Clin Exp Res. 2020 Dec;44(12):2519-2535. doi: 10.1111/acer.14482. Epub 2020 Nov 16. Alcohol Clin Exp Res. 2020. PMID: 33067812 Free PMC article.
References
-
- Aldridge V., Garg A., Davies N., Bartlett D. C., Youster J., Beard H., Kavanagh D. P., Kalia N., Frampton J., Lalor P. F, et al. (2012). Human mesenchymal stem cells are recruited to injured liver in a β1-integrin and CD44 dependent manner. Hepatology 56, 1063–1073 - PubMed
-
- Andrade M. C., Albernaz M. J., Araújo M. S., Santos B. P., Teixeira-Carvalho A., Faria A. M., Martins-Filho O. A. (2009). Short-term administration of ethanol in mice deviates antigen presentation activity towards B cells. Scand. J. Immunol. 70, 226–237 - PubMed
-
- Beamand J. A., Price R. J., Cunninghame M. E., Lake B. G. (1993). Culture of precision-cut liver slices: Effect of some peroxisome proliferators. Food Chem. Toxicol. 31, 137–147 - PubMed
-
- Bhogal R. H., Hodson J., Bartlett D. C., Weston C. J., Curbishley S. M., Haughton E., Williams K. T., Reynolds G. M., Newsome P. N., Adams D. H, et al. (2011). Isolation of primary human hepatocytes from normal and diseased liver tissue: A one hundred liver experience. PLoS ONE 6, e18222 - PMC - PubMed
-
- Boyadjieva N., Sarkar D. K. (1999). Effects of ethanol on basal and adenosine-induced increases in beta-endorphin release and intracellular cAMP levels in hypothalamic cells. Brain Res. 824, 112–118 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

