Ubiquitination by the membrane-associated RING-CH-8 (MARCH-8) ligase controls steady-state cell surface expression of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) receptor 1
- PMID: 23300075
- PMCID: PMC3585101
- DOI: 10.1074/jbc.M112.448209
Ubiquitination by the membrane-associated RING-CH-8 (MARCH-8) ligase controls steady-state cell surface expression of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) receptor 1
Abstract
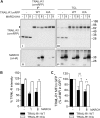
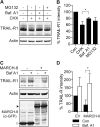
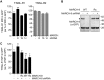
The eleven members of the membrane-associated RING-CH (MARCH) ubiquitin ligase family are relatively unexplored. Upon exogenous (over)expression, a number of these ligases can affect the trafficking of membrane molecules. However, only for MARCH-1 endogenous functions have been demonstrated. For the other endogenous MARCH proteins, no functions or substrates are known. We report here that TRAIL-R1 is a physiological substrate of the endogenous MARCH-8 ligase. Human TRAIL-R1 and R2 play a role in immunosurveillance and are targets for cancer therapy, because they selectively induce apoptosis in tumor cells. We demonstrate that TRAIL-R1 is down-regulated from the cell surface, with great preference over TRAIL-R2, by exogenous expression of MARCH ligases that are implicated in endosomal trafficking, such as MARCH-1 and -8. MARCH-8 attenuated TRAIL-R1 cell surface expression and apoptosis signaling by virtue of its ligase activity. This suggested that ubiquitination of TRAIL-R1 was instrumental in its down-regulation by MARCH-8. Indeed, in cells with endogenous MARCH expression, TRAIL-R1 was ubiquitinated at steady-state, with the conserved membrane-proximal lysine 273 as one of the potential acceptor sites. This residue was also essential for the interaction of TRAIL-R1 with MARCH-1 and MARCH-8 and its down-regulation by these ligases. Gene silencing identified MARCH-8 as the endogenous ligase that ubiquitinates TRAIL-R1 and attenuates its cell surface expression. These findings reveal that endogenous MARCH-8 regulates the steady-state cell surface expression of TRAIL-R1.
Figures
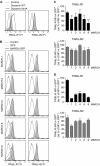
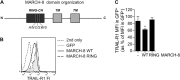
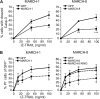
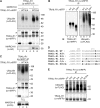

References
-
- Smyth M. J., Cretney E., Takeda K., Wiltrout R. H., Sedger L. M., Kayagaki N., Yagita H., Okumura K. (2001) Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon γ-dependent natural killer cell protection from tumor metastasis. J. Exp. Med. 193, 661–670 - PMC - PubMed
-
- Takeda K., Hayakawa Y., Smyth M. J., Kayagaki N., Yamaguchi N., Kakuta S., Iwakura Y., Yagita H., Okumura K. (2001) Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat. Med. 7, 94–100 - PubMed
-
- Kimberley F. C., Screaton G. R. (2004) Following a TRAIL. Update on a ligand and its five receptors. Cell Res. 14, 359–372 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

