Intracellular trafficking of the KV1.3 potassium channel is regulated by the prodomain of a matrix metalloprotease
- PMID: 23300077
- PMCID: PMC3585079
- DOI: 10.1074/jbc.M112.421495
Intracellular trafficking of the KV1.3 potassium channel is regulated by the prodomain of a matrix metalloprotease
Abstract
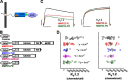
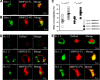
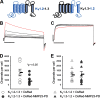
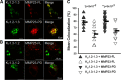
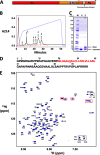
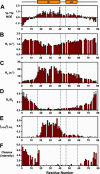
Matrix metalloproteases (MMPs) are endopeptidases that regulate diverse biological processes. Synthesized as zymogens, MMPs become active after removal of their prodomains. Much is known about the metalloprotease activity of these enzymes, but noncanonical functions are poorly defined, and functions of the prodomains have been largely ignored. Here we report a novel metalloprotease-independent, channel-modulating function for the prodomain of MMP23 (MMP23-PD). Whole-cell patch clamping and confocal microscopy, coupled with deletion analysis, demonstrate that MMP23-PD suppresses the voltage-gated potassium channel KV1.3, but not the closely related KV1.2 channel, by trapping the channel intracellularly. Studies with KV1.2-1.3 chimeras suggest that MMP23-PD requires the presence of the KV1.3 region from the S5 trans-membrane segment to the C terminus to modulate KV1.3 channel function. NMR studies of MMP23-PD reveal a single, kinked trans-membrane α-helix, joined by a short linker to a juxtamembrane α-helix, which is associated with the surface of the membrane and protected from exchange with the solvent. The topological similarity of MMP23-PD to KCNE1, KCNE2, and KCNE4 proteins that trap KV1.3, KV1.4, KV3.3, and KV3.4 channels early in the secretory pathway suggests a shared mechanism of channel regulation. MMP23 and KV1.3 expression is enhanced and overlapping in colorectal cancers where the interaction of the two proteins could affect cell function.
Figures


Similar articles
-
Domain structure and function of matrix metalloprotease 23 (MMP23): role in potassium channel trafficking.Cell Mol Life Sci. 2014 Apr;71(7):1191-210. doi: 10.1007/s00018-013-1431-0. Epub 2013 Aug 3. Cell Mol Life Sci. 2014. PMID: 23912897 Free PMC article. Review.
-
KCNE4 suppresses Kv1.3 currents by modulating trafficking, surface expression and channel gating.J Cell Sci. 2009 Oct 15;122(Pt 20):3738-48. doi: 10.1242/jcs.056689. Epub 2009 Sep 22. J Cell Sci. 2009. PMID: 19773357
-
Potassium channel modulation by a toxin domain in matrix metalloprotease 23.J Biol Chem. 2010 Mar 19;285(12):9124-36. doi: 10.1074/jbc.M109.071266. Epub 2009 Dec 4. J Biol Chem. 2010. PMID: 19965868 Free PMC article.
-
Kv1 potassium channel C-terminus constant HRETE region: arginine substitution affects surface protein level and conductance level of subfamily members differentially.Mol Membr Biol. 2007 May-Jun;24(3):194-205. doi: 10.1080/09687860601066309. Mol Membr Biol. 2007. PMID: 17520476
-
The secret life of ion channels: Kv1.3 potassium channels and proliferation.Am J Physiol Cell Physiol. 2018 Jan 1;314(1):C27-C42. doi: 10.1152/ajpcell.00136.2017. Epub 2017 Sep 20. Am J Physiol Cell Physiol. 2018. PMID: 28931540 Review.
Cited by
-
Ion channels and anti-cancer immunity.Philos Trans R Soc Lond B Biol Sci. 2014 Feb 3;369(1638):20130106. doi: 10.1098/rstb.2013.0106. Print 2014 Mar 19. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24493754 Free PMC article. Review.
-
Hic-5 regulates extracellular matrix-associated gene expression and cytokine secretion in cancer associated fibroblasts.Exp Cell Res. 2024 Feb 15;435(2):113930. doi: 10.1016/j.yexcr.2024.113930. Epub 2024 Jan 17. Exp Cell Res. 2024. PMID: 38237846 Free PMC article.
-
Domain structure and function of matrix metalloprotease 23 (MMP23): role in potassium channel trafficking.Cell Mol Life Sci. 2014 Apr;71(7):1191-210. doi: 10.1007/s00018-013-1431-0. Epub 2013 Aug 3. Cell Mol Life Sci. 2014. PMID: 23912897 Free PMC article. Review.
-
Structure of the voltage-gated potassium channel KV1.3: Insights into the inactivated conformation and binding to therapeutic leads.Channels (Austin). 2023 Dec;17(1):2253104. doi: 10.1080/19336950.2023.2253104. Channels (Austin). 2023. PMID: 37695839 Free PMC article. Review.
-
Melanoma expression of matrix metalloproteinase-23 is associated with blunted tumor immunity and poor responses to immunotherapy.J Transl Med. 2014 Dec 10;12:342. doi: 10.1186/s12967-014-0342-7. J Transl Med. 2014. PMID: 25491880 Free PMC article.
References
-
- Mannello F., Medda V. (2012) Nuclear localization of matrix metalloproteinases. Prog. Histochem. Cytochem. 47, 27–58 - PubMed
-
- Nagase H., Visse R., Murphy G. (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 69, 562–573 - PubMed
-
- Jozic D., Bourenkov G., Lim N. H., Visse R., Nagase H., Bode W., Maskos K. (2005) X-ray structure of human proMMP-1: new insights into procollagenase activation and collagen binding. J. Biol. Chem. 280, 9578–9585 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

