Identification of propofol binding sites in a nicotinic acetylcholine receptor with a photoreactive propofol analog
- PMID: 23300078
- PMCID: PMC3585054
- DOI: 10.1074/jbc.M112.435909
Identification of propofol binding sites in a nicotinic acetylcholine receptor with a photoreactive propofol analog
Abstract
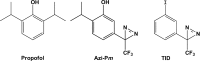
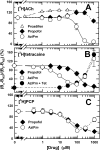
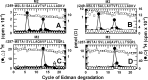
Propofol, a widely used intravenous general anesthetic, acts at anesthetic concentrations as a positive allosteric modulator of γ-aminobutyric acid type A receptors and at higher concentration as an inhibitor of nicotinic acetylcholine receptors (nAChRs). Here, we characterize propofol binding sites in a muscle-type nAChR by use of a photoreactive analog of propofol, 2-isopropyl-5-[3-(trifluoromethyl)-3H-diazirin-3-yl]phenol (AziPm). Based upon radioligand binding assays, AziPm stabilized the Torpedo nAChR in the resting state, whereas propofol stabilized the desensitized state. nAChR-rich membranes were photolabeled with [(3)H]AziPm, and labeled amino acids were identified by Edman degradation. [(3)H]AziPm binds at three sites within the nAChR transmembrane domain: (i) an intrasubunit site in the δ subunit helix bundle, photolabeling in the nAChR desensitized state (+agonist) δM2-18' and two residues in δM1 (δPhe-232 and δCys-236); (ii) in the ion channel, photolabeling in the nAChR resting, closed channel state (-agonist) amino acids in the M2 helices (αM2-6', βM2-6' and -13', and δM2-13') that line the channel lumen (with photolabeling reduced by >90% in the desensitized state); and (iii) at the γ-α interface, photolabeling αM2-10'. Propofol enhanced [(3)H]AziPm photolabeling at αM2-10'. Propofol inhibited [(3)H]AziPm photolabeling within the δ subunit helix bundle at lower concentrations (IC50 = 40 μm) than it inhibited ion channel photolabeling (IC50 = 125 μm). These results identify for the first time a single intrasubunit propofol binding site in the nAChR transmembrane domain and suggest that this is the functionally relevant inhibitory binding site.
Figures






Similar articles
-
Multiple transmembrane binding sites for p-trifluoromethyldiazirinyl-etomidate, a photoreactive Torpedo nicotinic acetylcholine receptor allosteric inhibitor.J Biol Chem. 2011 Jun 10;286(23):20466-77. doi: 10.1074/jbc.M111.219071. Epub 2011 Apr 15. J Biol Chem. 2011. PMID: 21498509 Free PMC article.
-
Enantiomeric barbiturates bind distinct inter- and intrasubunit binding sites in a nicotinic acetylcholine receptor (nAChR).J Biol Chem. 2017 Oct 20;292(42):17258-17271. doi: 10.1074/jbc.M117.808592. Epub 2017 Sep 6. J Biol Chem. 2017. PMID: 28878016 Free PMC article.
-
Multiple propofol-binding sites in a γ-aminobutyric acid type A receptor (GABAAR) identified using a photoreactive propofol analog.J Biol Chem. 2014 Oct 3;289(40):27456-68. doi: 10.1074/jbc.M114.581728. Epub 2014 Aug 1. J Biol Chem. 2014. PMID: 25086038 Free PMC article.
-
Anesthetics target interfacial transmembrane sites in nicotinic acetylcholine receptors.Neuropharmacology. 2015 Sep;96(Pt B):169-77. doi: 10.1016/j.neuropharm.2014.10.002. Epub 2014 Oct 12. Neuropharmacology. 2015. PMID: 25316107 Free PMC article. Review.
-
Functional domains of the nicotinic acetylcholine receptor.Ann N Y Acad Sci. 1986;463:53-69. doi: 10.1111/j.1749-6632.1986.tb21503.x. Ann N Y Acad Sci. 1986. PMID: 3521435 Review.
Cited by
-
Shedding Light on Anesthetic Mechanisms: Application of Photoaffinity Ligands.Anesth Analg. 2016 Nov;123(5):1253-1262. doi: 10.1213/ANE.0000000000001365. Anesth Analg. 2016. PMID: 27464974 Free PMC article. Review.
-
Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human α1β3γ2 γ-aminobutyric acid type A (GABAA) receptor.J Biol Chem. 2013 Jul 5;288(27):19343-57. doi: 10.1074/jbc.M113.479725. Epub 2013 May 15. J Biol Chem. 2013. PMID: 23677991 Free PMC article.
-
Ethanol Modulation is Quantitatively Determined by the Transmembrane Domain of Human α1 Glycine Receptors.Alcohol Clin Exp Res. 2015 Jun;39(6):962-8. doi: 10.1111/acer.12735. Epub 2015 May 14. Alcohol Clin Exp Res. 2015. PMID: 25973519 Free PMC article.
-
Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel.Nat Commun. 2013;4:1697. doi: 10.1038/ncomms2682. Nat Commun. 2013. PMID: 23591864 Free PMC article.
-
Intravenous Anesthetic Protects Hepatocyte from Reactive Oxygen Species-Induced Cellular Apoptosis during Liver Transplantation In Vivo.Oxid Med Cell Longev. 2018 Oct 28;2018:4780615. doi: 10.1155/2018/4780615. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30510620 Free PMC article.
References
-
- Krasowski M. D., Jenkins A., Flood P., Kung A. Y., Hopfinger A. J., Harrison N. L. (2001) General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of γ-aminobutyric acid (GABA) current at the GABA(A) receptor but not with lipid solubility. J. Pharmacol. Exp. Ther. 297, 338–351 - PubMed
-
- Rudolph U., Antkowiak B. (2004) Molecular and neuronal substrates for general anaesthetics. Nat. Rev. Neurosci. 5, 709–720 - PubMed
-
- Franks N. P. (2008) General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 9, 370–386 - PubMed
-
- Flood P., Ramirez-Latorre J., Role L. (1997) α4β2 neuronal nicotinic acetylcholine receptors in the central nervous system are inhibited by isoflurane and propofol, but α7-type nicotinic acetylcholine receptors are unaffected. Anesthesiology 86, 859–865 - PubMed
-
- Violet J. M., Downie D. L., Nakisa R. C., Lieb W. R., Franks N. P. (1997) Differential sensitivities of mammalian neuronal and muscle nicotinic acetylcholine receptors to general anesthetics. Anesthesiology 86, 866–874 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

